
 | |
| Volume 10, Number 9 | May 19, 2020 |
 |
It is curious, the things the mundane discipline of biblical chronology can get a scientist into.
Two decades ago, I embarked on a full-time quest to discover why humans had lived so much longer before Noah's Flood than they do today.[1] My chronology research, combining radiocarbon dating and biblical chronology, had revealed a missing thousand years in traditional biblical chronologies such as the Ussher chronology.[2] Prior to its discovery, this missing thousand years had caused biblical chronology to be seriously out of step with radiocarbon and secular historical chronologies prior to about 1000 B.C., and this had made the biblical historical narrative from Genesis through Judges seem unhistorical.[3] When the missing thousand years were restored to their rightful place in biblical chronology, biblical and secular chronologies were found to be in harmony from the present back into the earliest chapters in Genesis. Noah's Flood, for example, recounted in Genesis chapters 6 through 9, was found to have a radiocarbon date of 3525±12.5 B.C. and a biblical chronology date of 3520±21 B.C.[4] These two dates are breathtakingly indistinguishable for such a remote time.
Once it has become clear that biblical chronology is highly dependable back into the earliest chapters in Genesis, then it immediately follows that the multi-century life spans of humans recorded in the early chapters in Genesis must be factual, for the genealogies in which these life spans are found in Genesis 5 and Genesis 11 provide the backbone for biblical chronology for more than two thousand years, from before Noah's Flood until well after it. This, of course, leads immediately to the question of why ordinary people lived so much longer back then than we do now.
I conjectured that pre-Flood humans were getting a vitamin that we no longer get today, and that aging as we know it is a deficiency disease of this vitamin. I called the unknown vitamin "vitamin X," and I set out to find it.[5]
The present article analyzes research carried out using ICR[6] female weanling mice in the ARP Rodent Lab between March 2001 and September 2019 to discover vitamin X. Four separate, same-age cohorts of mice of that type (in addition to numerous other cohorts of other types of mice) were used during those eighteen and a half years to test a variety of vitamin X candidate substances both for toxicity and for life-lengthening efficacy. The choice of vitamin X candidates was guided by a constantly maturing theory explaining biblically-recorded, near-1,000-year human life spans prior to Noah's Flood.[7] Sister vitamin X candidates, methylphosphonic acid (MePA) and methylphosphinic acid (MePiA), were tested separately with the fourth cohort of ICR female weanling mice. By the time this MePA-versus-MePiA test was initiated, both the theoretical side and the experimental side of the work had progressed sufficiently to predict with near certainty that either MePA or MePiA or both were the long-sought vitamin X. This prediction had received preliminary confirmation by dramatic, positive health effects due to self-dosing with 1 μg MePA per day.[8]
The working hypothesis at the launching of the MePA-vs-MePiA experiment was that MePA and MePiA acted as a pair of vitamins in the body. Experiments on mice treated with MePA had shown no life-lengthening. The working hypothesis was that this was due to mice lacking the enzymes necessary to convert MePA to MePiA. The MePA-vs-MePiA experiment was designed to test this hypothesis. MePiA, according to the working hypothesis, acts as an anti-oxidant in the body to mitigate damage due to free radicals, in accordance with Harman's free radical theory of aging.[9] MePiA is oxidized to MePA by free radicals of the reactive oxygen species group including OH·, the hydroxyl radical. MePA may then operate in a typical vitamin capacity in a variety of biochemical reactions. Ultimately, it is either enzymatically reduced back to MePiA or filtered by the kidneys and excreted from the body. This working hypothesis makes MePiA to be essential for life lengthening, and the expectation was that mice receiving MePiA would show life lengthening.
Life span experiments with mice take several years to run to completion. I have previously described how early results from this experiment seemed contrary to the hypothesis, leaving MePA as the sole anti-aging vitamin, and how it took two years for this mistaken impression to be corrected.[10] The present article shows that the final results of the experiment corroborate the working hypothesis beyond reasonable doubt. It shows that the mice receiving MePiA did experience life lengthening. Of the 16 vitamin X candidates tested with these four cohorts of mice, and of however many other vitamin X candidates tested with other types of mice in the ARP Rodent Lab, only MePiA has ever given a positive, life-lengthening signal.
The 2019 discovery of the life-lengthening efficacy of MePiA with mice was announced in Addendum to Aging: Cause and Cure.[11] Three MePiA-treated mice played an especially important role in that publication.[12] These three mice were all unusually long lived. Of these three mice, one (subsequently dubbed "ELLM") was of special interest. ELLM appeared more youthful than the other two, both of whom appeared quite aged, and she outlived the other two. At the time of writing of Addendum to Aging: Cause and Cure, the two aged-looking mice had died and ELLM was still alive.
ELLM died seven and a half weeks later. She, too, looked aged by the time she died. She was 171.6 weeks of age when she died. This is an extraordinarily long life span for this type of mouse. The previous record age for this same type of mouse (ICR female weanling) in ARP Rodent Lab, not treated with MePiA, was 150.4 weeks. This record is for a total of 176 mice of this type raised in ARP Lab. ELLM extended this record by 21.2 weeks (14%).
In human-equivalent terms, ELLM lived for 135.3 years.[13] The previous record (150.4 weeks) mouse had a human-equivalent age of 118.6 years. The world record age for modern humans—from a sample population numbered in the billions—is 122.4 years.[14] In human-equivalent terms, ELLM outlived the human record age by nearly 15 years. She was truly extraordinarily long lived—which is why she was dubbed "ELLM," an acronym for Extraordinarily Long Lived Mouse.
ELLM's long life makes an important contribution to the all-important question of whether treatment of her group with MePiA irrefutably demonstrates life lengthening. Addendum to Aging: Cause and Cure relied upon a simple graphical presentation of survival curve data to make the case that it had done so. The present article performs a quantitative, mathematical analysis of the data, with the final data point for ELLM's group (resulting from her death) included, to make the same case more objectively and more forcefully.
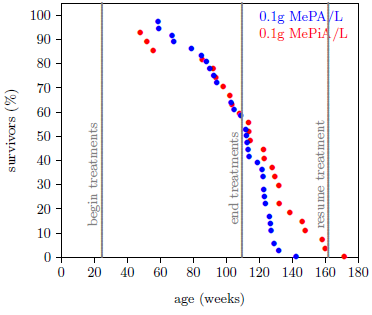
Figure 2 shows the final graph of the MePA-vs-MePiA dataset. Each treatment group began with 4 cages containing 9 mice each, all of the same age. One cage was removed from the MePiA dataset due to a treatment-unrelated, juvenile die-off event as previously discussed.[15] The final red dot on the right corresponds to the ELLM datapoint.
 |
In BC107, a means of analyzing survival curve data in light of the general theory of aging was introduced.[16] A weighted least-squares method is used to fit the Aardsma model (Equation 1) to experimental datasets.
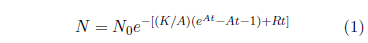
The Aardsma model has three potentially free parameters: A, K, and R. It was pointed out in BC108 that A may be treated as a fixed parameter when the model is used to compare survival curves for organisms of a given type.[17] This reduces the model from three to two parameters. The two-parameter model simplifies interpretation of the fit. Specifically, whatever changes in aging there may be within the datasets being compared will show up as changes to K. Since all of the survival curves of interest this issue are for ICR female weanling mice, it is expedient to use the two-parameter model.
To determine the value of A for ICR female weanling mice, datasets from four cohorts were used. These four cohorts are all of this type of mouse ever used in the ARP Rodent Lab. For the first three cohorts, the treated and control mice in each cohort were pooled to produce a survival curve for that cohort. The fourth cohort was split into the two groups depicted in Figure 2. This split was necessary because of the difference in longevity between MePA-treated and MePiA-treated mice. This produced five independent datasets for ICR female weanling mice (Figure 3).
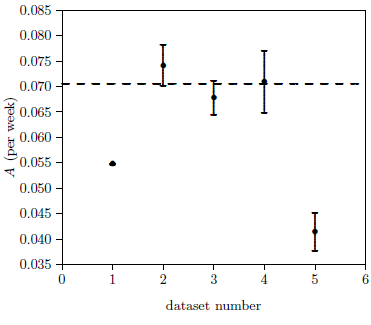 |
A three-parameter, weighted least-squares fit of the Aardsma model was performed with these five datasets. Of the five A values obtained, three were grouped (dataset numbers 2, 3, and 4) while two appeared as separate outliers. Because A is expected to be the same for organisms of a given type, a weighted average of the three grouped values was used to determine A for the two-parameter model. This gave A = 0.070517, shown as the dashed line in Figure 3.
Two-parameter fits of the Aardsma model to the five datasets were then performed with A treated as a fixed parameter having a value of 0.070517. The resulting K values are shown in Figure 4.
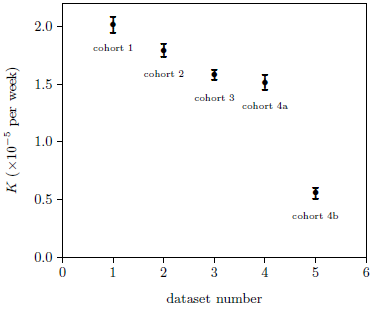 |
As K measures aging effects, the K values for the first four datasets were expected to be similar. Surprisingly, the first three datasets show a slow, apparently real decline in K. This implies a slow, small increase in life spans for these first three cohorts.
It is difficult to see how this slow drift in life spans could be due to treatments or environmental conditions in the ARP Lab. Rather, it seems probably to represent what I will call "stock drift." Stock drift includes, for example, genetic change or improved removal of viruses affecting the breeder's ICR stock. This explanation is encouraged by the length of time which elapsed between birthdates of the four cohorts (Figure 5). A simple linear drift with time adequately describes the changes observed in these four K values.
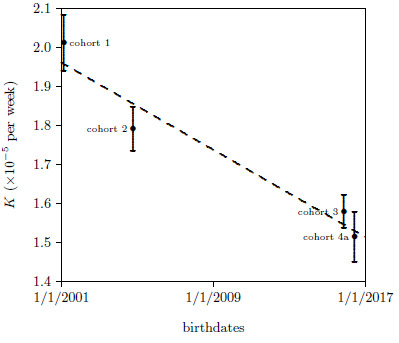 |
Figure 4 shows a dramatic separation in K values between the MePiA-treated group (dataset number 5, cohort 4b) and the other four datasets from mice not treated with MePiA. The separation in these two K values is so large relative to their error bars that no formal statistical analysis is needed to reject the null hypothesis and conclude with an extremely high level of confidence that the K value for dataset number 5 (cohort 4b) is truly less than the K value for dataset number 4 (cohort 4a). Since K "seems to be all about longevity,"[18] this says, with extreme confidence, that the longevity of the 4b mice was significantly increased relative to the 4a mice. Figure 6 shows this graphically.
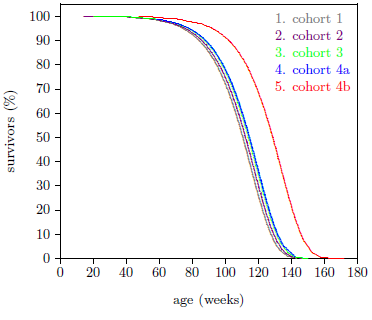 |
The only remaining question is whether the observed increase in longevity is due to MePiA.
The fact that the K value for dataset number 4 (for the MePA-treated control group, cohort 4a) is statistically indistinguishable from the K value for dataset number 3 (cohort 3; see Figure 5) shows that there was nothing unusual about cohort 4 mice.
Since both dataset number 4 (the control group) and dataset number 5 (the treatment group) used cohort 4 mice, no component of the large separation in K values between dataset number 4 and dataset number 5 is due to stock drift.
Finally, notice again that, even though MePiA is expected to be slowly converted to MePA in vivo, no component of the large separation in K values between 4 and 5 may be attributed to MePA as the control group (4a) was treated with MePA.
The conclusion that MePiA treatment of the dataset number 5 mice is the sole cause of their increased longevity appears to be robust.
In the last issue, I introduced the two-phase theory of human aging.[19] This theory distills modern human aging down into three separate diseases:
Aging 0: congenital vitamin MePA deficiency disease,
Aging 1: congenital vitamin MePiA deficiency disease, and
Aging 2: a mitochondrial genetic disease induced by Aging 1.
So far, mouse experiments have failed to show any life lengthening due to MePA. This does not mean that mice are not subject to Aging 0, however. The problem is that Aging 1 is the dominant deficiency disease, making any Aging 0 effects on mouse survival curves difficult to detect. It seems likely that the impact of Aging 0 on mice will not be known until a deliberate experiment, probably involving a very large number of mice, is run to find out. This experiment will need to be run in the absence of dietary MePiA since MePiA oxidizes to produce MePA. In other words, it will need to be run on mice who are afflicted with the dominant Aging 1 disease, which will continue to make MePA-specific effects difficult to detect. Because of the urgent need to know more about Aging 1 and Aging 2, this experiment is likely to be of low priority and is probably some years off.
Because MePiA lengthens life spans of mice, it is clear that mice are subject to Aging 1. From a theoretical perspective, this is not very surprising. It is really just saying that mice suffer free radical tissue damage which MePiA ameliorates. Aging 1 in mice is of great interest at present and seems sure to be intensely studied going forward. Looming questions at present include the effect on longevity of MePiA treatments at different concentrations, and the effect on longevity of beginning MePiA treatment at different ages.
Work with progeroid mice seems to imply that mice are subject to Aging 2.[20] These mice have an artificially created mtDNA repair defect which causes them to show symptoms of aging earlier than usual and to die sooner than usual. This artificial mtDNA repair defect in mice seems to be simply a way of hastening what free radical damage of mtDNA accomplishes in humans, namely, dysfunctional scrapping of damaged mtDNA, which equates to induction of Aging 2.[21] Thus, symptoms of aging in normal mice, including aging death, appear to be due to Aging 2.
Since mice appear to be subject to Aging 1 and Aging 2, they should serve as useful experimental models for further research into human aging, allowing such urgent questions as the efficacy of MePiA for combating Aging 2 to be answered. Specifically, if Aging 2 is present in mice and not responsive to MePiA, then a graph of life lengthening versus MePiA treatment starting age should decline suddenly at the age of onset of Aging 2. If Aging 2 is responsive to MePiA in mice, then no sudden decline should be seen. Figure 6 suggests that the age of onset for Aging 2 is approximately one year for ICR female weanling mice. Treatment of the cohort 4 mice began just prior to half a year of age (Figure 2), seemingly well before Aging 2 had begun.
The quest to find vitamin X is now over. MePiA has successfully increased longevity in ICR female weanling mice. The extraordinarily long life span of ELLM makes her to be Exhibit A of this result (Figure 1), but she does not stand alone. The least-squares method includes all 27 mice of her treated group in its determination of K, and it is the reduction in K relative to the control group which produces extreme (off the statistics charts) confidence in this result.
There is still much to learn. At present, we don't know the efficacious treatment concentration range of MePiA in mice. We don't know how much longer mice may live if treatments are begun at younger ages. We don't know whether mice are genetically equipped to make best use of MePiA the way humans appear to be. What we have at this stage are two undeniable facts: (1) pre-Flood humans lived very much longer than modern humans, and (2) mice whose diets have been supplemented with MePiA live longer than mice who have not been supplemented with MePiA. These two facts find a common explanation in the hypothesis that MePiA is a previously unknown exogenous antioxidant vitamin, the lack of which causes a vitamin deficiency disease, Aging 1, leading to premature death.
Noah's Flood reduced the natural source of MePiA effectively to zero.[22] Five and a half thousand years later, we are yet living in the shadow of that global geophysical catastrophe[23] so that there remains today no functional natural source of vitamin MePiA. To halt the ravages of Aging 1, it is necessary to supplement one's diet with this vitamin artificially.
Synchronous with the publication of Addendum to Aging: Cause and Cure, I made available as a dietary supplement Dr. Aardsma's Anti-Aging Vitamins. This supplement contains both MePiA and MePA.[24] The two-phase theory of human aging says that those who have not started taking this supplement will have Aging 0 disease, Aging 1 disease, and, if old enough, Aging 2 disease. If they continue not taking this supplement, they will age and die around 70 or 80 years of age as humans have been aging and dying for some four and a half thousand years now. Those who take the supplement will halt and cure Aging 0 disease, and they will halt and cure Aging 1 disease. If they started taking the supplement at a young enough age, they will not contract Aging 2 disease. Their life expectancy will then be on the order of ten thousand years, as previously calculated.[25] If it is found to be the case that MePiA is effective against Aging 2, then extended life expectancies will also apply to individuals who started supplementing with the anti-aging vitamin duo after they had already contracted Aging 2. Any individuals who stop supplementing their diets with the anti-aging vitamins will contract Aging 0 and Aging 1 again, just as surely as they would contract scurvy if they eliminated vitamin C from their diets, and they will close the door to the only known hope at present of combating Aging 2 disease.
To maximize health and longevity, Rules 1 and 2 still apply:[26]
Rule 1: Whatever your age, begin taking the vitamins without delay.
Rule 2: Take the vitamins without fail every day. ◇
The Biblical Chronologist is written and edited by Gerald E. Aardsma, a Ph.D. scientist (nuclear physics) with special background in radioisotopic dating methods such as radiocarbon. The Biblical Chronologist has a fourfold purpose: to encourage, enrich, and strengthen the faith of conservative Christians through instruction in biblical chronology and its many implications, to foster informed, up-to-date, scholarly research in this vital field, to communicate current developments and discoveries stemming from biblical chronology in an easily understood manner, and to advance the growth of knowledge via a proper integration of ancient biblical and modern scientific data and ideas. The Biblical Chronologist (ISSN 1081-762X) is published by: Aardsma Research & Publishing Copyright © 2020 by Aardsma Research & Publishing.
|
^ Gerald E. Aardsma, "Research in Progress," The Biblical Chronologist 8.6 (November/December 2002): 15–16. www.BiblicalChronologist.org.
^ Gerald E. Aardsma, A New Approach to the Chronology of Biblical History from Abraham to Samuel, 2nd ed. (Loda, IL: Aardsma Research and Publishing, 1995). www.BiblicalChronologist.org.
^ Gerald E. Aardsma, "Evidence for a Lost Millennium in Biblical Chronology," Radiocarbon 37.2 (1995): 267–273.
^ Gerald E. Aardsma, Noah's Flood Happened 3520 B.C. (Loda, IL: Aardsma Research and Publishing, 2015), pages 307–313. www.BiblicalChronologist.org.
^ Gerald E. Aardsma, "The Cause of Reduced Post-Flood Life Spans – Part IV," The Biblical Chronologist 8.1 (January/February 2002): 1–8. www.BiblicalChronologist.org.
^ These are outbred white laboratory mice. The full designation of the strain is Hsd:ICR (CD-1®).
^ Gerald E. Aardsma, Aging: Cause and Cure (Loda, IL: Aardsma Research and Publishing, 2017). www.BiblicalChronologist.org.
^ Gerald E. Aardsma, Aging: Cause and Cure (Loda, IL: Aardsma Research and Publishing, 2017), pages 113–118. www.BiblicalChronologist.org.
^ Denham Harman, "The biologic clock: the mitochondria?," Journal of the American Geriatrics Society 20 (1972): 145–147.
^ Gerald E. Aardsma, Addendum to Aging: Cause and Cure (Loda, IL: Aardsma Research and Publishing, July 26, 2019), pages 2–5. www.BiblicalChronologist.org.
^ Gerald E. Aardsma, Addendum to Aging: Cause and Cure (Loda, IL: Aardsma Research and Publishing, July 26, 2019). www.BiblicalChronologist.org.
^ Gerald E. Aardsma, Addendum to Aging: Cause and Cure (Loda, IL: Aardsma Research and Publishing, July 26, 2019), pages 4, 8, and 10. www.BiblicalChronologist.org.
^ I have used the Social Security Administration's 2016 Actuarial Life Table (ssa.gov/oact/STATS/table4c6.html) to calculate this human-equivalent age. Specifically, the 50% survivors point for U.S. females in 2016 is 84.6 years. For the cohort of mice sporting the previous 150.4 week record, the 50% survivors point is 107.3 weeks. This yields (171.6×84.6/107.3=) 135.3 years.
^ en.wikipedia.org/wiki/Oldest People (accessed May 18, 2020).
^ Gerald E. Aardsma, Addendum to Aging: Cause and Cure (Loda, IL: Aardsma Research and Publishing, July 26, 2019), pages 6–9. www.BiblicalChronologist.org.
^ Gerald E. Aardsma, "Modeling Survival Curves in Light of the General Theory of Aging," The Biblical Chronologist 10.7 (April 24, 2020): 1–7. www.BiblicalChronologist.org.
^ Gerald E. Aardsma, "Human Aging is a Two-Phase Disease," The Biblical Chronologist 10.8 (May 13, 2020): 4. www.BiblicalChronologist.org.
^ Gerald E. Aardsma, "Human Aging is a Two-Phase Disease," The Biblical Chronologist 10.8 (May 13, 2020): page 4. www.BiblicalChronologist.org.
^ Gerald E. Aardsma, "Human Aging is a Two-Phase Disease," The Biblical Chronologist 10.8 (May 13, 2020): 1–10. www.BiblicalChronologist.org.
^ R. H. Hämäläinen, J. C. Landoni, K. J. Ahlqvist, et al. "Defects in mtDNA replication challenge nuclear genome stability through nucleotide depletion and provide a unifying mechanism for mouse progerias," Nature Metabolism 1.10 (1 October 2019): pages 958-965.
^ Gerald E. Aardsma, "Human Aging is a Two-Phase Disease," The Biblical Chronologist 10.8 (May 13, 2020): 7–8. www.BiblicalChronologist.org.
^ Gerald E. Aardsma, Aging: Cause and Cure (Loda, IL: Aardsma Research and Publishing, 2017), pages 67–70. www.BiblicalChronologist.org.
^ Gerald E. Aardsma, Noah's Flood Happened 3520 B.C. (Loda, IL: Aardsma Research and Publishing, 2015). www.BiblicalChronologist.org.
^ Dr. Aardsma's Anti-Aging Vitamins can be obtained via AgingCauseAndCure.com/get-vitamin-mepa/.
^ Gerald E. Aardsma, "Effect of the Anti-Aging Vitamins on Life Expectancy Today," The Biblical Chronologist 10.6 (April 7, 2020): 1–8. www.BiblicalChronologist.org.
^ Gerald E. Aardsma, "Effect of the Anti-Aging Vitamins on Life Expectancy Today," The Biblical Chronologist 10.6 (April 7, 2020): 6–7. www.BiblicalChronologist.org.