
 |
 |
All rights reserved. No part of this publication may be reproduced, stored in a retrieval system or transmitted in any form or by any means: electronic, electrostatic, magnetic tape, mechanical, photocopying, recording or otherwise, without prior written permission of the publisher.
Published by:
Aardsma Research & Publishing
301 East Jefferson Street
Loda, Illinois 60948-8608
www.BiblicalChronologist.org
Scripture quotations taken from the (NASB®) New American Standard Bible®, Copyright© 1960, 1971, 1977, 1995 by The Lockman Foundation. Used by permission. All rights reserved. www.Lockman.org
Printed in the United States of America
Library of Congress Control Number: 2023901055
ISBN 978-1-7372151-2-7
| List of Tables | 7 |
| List of Figures | 9 |
| Dedication | 11 |
| Acknowledgments | 13 |
| 1 Introduction | 15 |
| 2 The Biblical Historical Record of Manna | 19 |
| 3 Constructing a Theory of Manna | 23 |
| 3.1 A Pause for Piety | 24 |
| 3.2 The Sorry State of Natural Explanations of Manna to the Present Time | 27 |
| 3.3 A Better Way | 30 |
| 3.3.1 Sabbath Air | 30 |
| 3.3.2 The Mystery Begins to Unravel | 34 |
| 3.3.3 The Stockyard Gases Theory of Manna | 35 |
| 3.4 Conclusion | 35 |
| 4 A First Attempt at Making Manna | 37 |
| 4.1 The Affinity of Ammonia for Water | 38 |
| 4.2 Acid-Base Reactions and Manna | 39 |
| 4.2.1 Acetic Acid | 39 |
| 4.2.2 Propionic Acid | 41 |
| 4.2.3 Butyric Acid | 44 |
| 4.3 Conclusion | 44 |
| 5 Improving the Stockyard Gases Theory of Manna | 47 |
| 5.1 Including the Desert | 47 |
| 5.1.1 Desert Soil Salts | 48 |
| 5.2 Efflorescence | 49 |
| 5.3 Conclusion | 51 |
| 6 Back to Making Manna | 53 |
| 6.1 Central Negev Cations | 54 |
| 6.1.1 The Dominance of Sodium in Cation Exchange with Ammonium | 54 |
| 6.2 Sodium and Acetate | 55 |
| 6.2.1 The Melting Point of Sodium Acetate Trihydrate | 56 |
| 6.2.2 The Taste of Sodium Acetate Trihydrate | 58 |
| 6.3 Other Ingredients | 60 |
| 6.3.1 Organic Acids | 60 |
| 6.3.2 Miscellaneous Trace Substances | 60 |
| 6.3.3 Sodium Hydroxide | 61 |
| 6.4 Recipe for Manna Synthesis | 61 |
| 6.5 Residual Evaluations | 63 |
| 6.5.1 Bdellium | 64 |
| 6.5.2 Foul Deductions | 66 |
| 6.6 Conclusion | 67 |
| 7 Further Confirmation: Manna Nutrition | 69 |
| 7.1 The Provision of Manna | 70 |
| 7.2 The Food Category of Manna | 71 |
| 7.3 Like Coriander Seed | 72 |
| 7.4 Manna Calories | 74 |
| 7.5 Conclusion | 75 |
| 8 Further Confirmation: Long-Term Manna Consumption | 77 |
| 8.1 Sodium Consumption | 78 |
| 8.2 Conclusion | 80 |
| 9 Conclusion | 81 |
| 9.1 Panoramic Summary | 81 |
| 9.2 The Mastermind | 82 |
| Appendices | 87 |
| A Negev Desert Climate at the Time of the Exodus | 89 |
| B Mathematical Model for Manna Production | 93 |
| B.1 Problem | 93 |
| B.2 Solution | 93 |
| B.2.1 References | 93 |
| B.2.2 Concentrations in Dew at the Surface of the Ground | 94 |
| B.2.3 Concentrations in Manna Solution | 99 |
| B.3 Conclusion | 101 |
| Index | 103 |
| 3.1 Some biblical observational data regarding manna. | 29 |
| 3.1 Artist's conception of an Israelite encampment. | 33 |
| 4.1 Temperature of 105°F recorded in the Negev desert. | 40 |
| 4.2 Ammonium propionate crystals made in the lab. | 41 |
| 4.3 Dried ammonium propionate crystals. | 43 |
| 5.1 Efflorescence on brickwork. | 50 |
| 6.1 Sodium acetate trihydrate spoilage test. | 57 |
| 6.2 Melting point apparatus. | 59 |
| 6.3 Manna manufactured in the lab. | 63 |
| 6.4 Bdellium compared to manna. | 64 |
| 6.5 Hoarfrost on a fencepost. | 65 |
| 7.1 Coriander seed compared to manna. | 73 |
| 9.1 Manna production. | 83 |
| A.1 Elevation of the surface of the Dead Sea. | 91 |
To all who would deny the historical accuracy of the biblical Exodus narrative. Checkmate.
To the billions of earth's inhabitants, in hopes that you may know our planet's real history and thereby be freed from the oppression of aging and other tyrannies.
I wish to express my sincere appreciation to those who assisted me with the production of this book.
Tamara Aardsma painted the illustration which appears in Figure 3.1.
Steve Hall assisted with graphic design, produced the covers and the illustration which appears in Figure 9.1, and shepherded the final draft through the printing process.
Tom Godfrey requires special mention. As proofreader, he provided copious high-quality feedback of all sorts on multiple drafts, including several comments originating with his wife, Beth. In addition, he crafted the index.
The manna mystery is succinctly stated in the historical record of manna's first appearance.
When the sons of Israel saw it, they said to one another, "What is it?" (Exodus 16:15.)
Here is a mystery which has stood unsolved for well over four thousand years. It dates back to 2450 B.C.[1] when the Israelites, miraculously delivered from slavery in Egypt, camped in the desert on their way from Egypt to Canaan.
This book will show you the solution to this mystery. By the time you have finished reading it, you will know what physical substance manna is, and you will be shown how you can view and handle and taste for yourself what millions of Israelites ate in the desert for forty years long ago.
I am a scientist with a PhD in physics. My specialty is physical dating methods, especially radiocarbon dating. I began to apply radiocarbon dating to problems in traditional biblical chronologies about thirty-five years ago. I have been researching full time at the interface of science and the Bible ever since. The solution to the manna mystery is the latest in a string of discoveries resulting from this Bible/science research.
Motivation to solve the mystery of manna came about as a result of an earlier Bible/science discovery presented in a previous book, Aging: Cause and Cure.[2] I set about to solve the mystery of manna to demonstrate as clearly as possible with manna something which is quite important in regard to aging but which will take decades to demonstrate with aging—that my Bible/science method leads to real solutions to age-old mysteries.
To demonstrate the validity of my Bible/science solution to the mystery of aging in a way that the average lay person can understand and trust requires keeping someone alive significantly longer than 122 years, the current record for human longevity in modern times. To make the point irrefutably, this record needs to be broken by several decades. This will happen—it is presently in process—but we must wait decades to get someone from his present age out several decades past 122 years of age. In sharp contrast, the solution to the manna mystery is immediately, conspicuously, obviously true—as this book will show.
I use the same Bible/science method in all my work. To solve the mystery of aging, for example, observations about human aging recorded in the Bible—especially the Bible's record of ancient life spans—were simply and unapologetically accepted at face value and analyzed using modern scientific principles and procedures to deduce the cause and the cure of aging. In the present book, observations about manna recorded in the Bible are simply and unapologetically accepted at face value and analyzed using modern scientific principles and procedures to deduce the physical substance corresponding to manna.
Now, there is no urgent reason why you need to trust what this method reveals about manna. The urgency lies entirely with what it has revealed about aging. It has revealed that what we call human "aging" is really just a disease resulting from dietary deficiency of two previously unknown vitamins, gradually lost from earth's environment in remote antiquity due to Noah's Flood. That is why people lived so much longer before the Flood than we do today. The two newly discovered vitamins are known chemical compounds which can easily be added back into the diet as supplements, just as can be done with any of the traditional vitamins. Individuals taking these new vitamins have experienced numerous significant health benefits which appear to have put them on a track to increased longevity.
Simply stated, the solution to the mystery of manna is revealed in this book using the same Bible/science method which was used to reveal the solution to the mystery of aging because I am greatly concerned that you should know now, without having to wait decades for someone to live well beyond 122 years, that the cure for aging has indeed been found. Aging is a debilitating, disfiguring, deadly disease. If left unattended, it will kill you—frequently after extensive medical interventions and nursing home/hospital residence. But the tide has turned. The cure has been found.[3] This book has been written to assure individuals having sufficient common sense to protect themselves from deadly diseases that this is indeed the case. My prayer is that this little book, which is all about manna and not at all about aging, will nonetheless be instrumental in saving the lives of millions from the disease we call aging.
The primary record of manna is found in chapter 16 of the biblical book of Exodus. It helps to know, while reading it, that an omer is a unit of measure roughly equal to a modern gallon,[4] so each time you see "omer" think "gallon."
1Then they set out from Elim, and all the congregation of the sons of Israel came to the wilderness of Sin, which is between Elim and Sinai, on the fifteenth day of the second month after their departure from the land of Egypt. 2The whole congregation of the sons of Israel grumbled against Moses and Aaron in the wilderness. 3The sons of Israel said to them, "Would that we had died by the Lord's hand in the land of Egypt, when we sat by the pots of meat, when we ate bread to the full; for you have brought us out into this wilderness to kill this whole assembly with hunger."4Then the Lord said to Moses, "Behold, I will rain bread from heaven for you; and the people shall go out and gather a day's portion every day, that I may test them, whether or not they will walk in My instruction. 5On the sixth day, when they prepare what they bring in, it will be twice as much as they gather daily." 6So Moses and Aaron said to all the sons of Israel, "At evening you will know that the Lord has brought you out of the land of Egypt; 7and in the morning you will see the glory of the Lord, for He hears your grumblings against the Lord; and what are we, that you grumble against us?"
8Moses said, "This will happen when the Lord gives you meat to eat in the evening, and bread to the full in the morning; for the Lord hears your grumblings which you grumble against Him. And what are we? Your grumblings are not against us but against the Lord."
9Then Moses said to Aaron, "Say to all the congregation of the sons of Israel, 'Come near before the Lord, for He has heard your grumblings.' " 10It came about as Aaron spoke to the whole congregation of the sons of Israel, that they looked toward the wilderness, and behold, the glory of the Lord appeared in the cloud. 11And the Lord spoke to Moses, saying, 12"I have heard the grumblings of the sons of Israel; speak to them, saying, 'At twilight you shall eat meat, and in the morning you shall be filled with bread; and you shall know that I am the Lord your God.' "
13So it came about at evening that the quails came up and covered the camp, and in the morning there was a layer of dew around the camp. 14When the layer of dew evaporated, behold, on the surface of the wilderness there was a fine flake-like thing, fine as the hoarfrost on the ground. 15When the sons of Israel saw it, they said to one another, "What is it?" For they did not know what it was. And Moses said to them, "It is the bread which the Lord has given you to eat. 16This is what the Lord has commanded, 'Gather of it every man as much as he should eat; you shall take an omer apiece according to the number of persons each of you has in his tent.' " 17The sons of Israel did so, and some gathered much and some little. 18When they measured it with an omer, he who had gathered much had no excess, and he who had gathered little had no lack; every man gathered as much as he should eat. 19Moses said to them, "Let no man leave any of it until morning." 20But they did not listen to Moses, and some left part of it until morning, and it bred worms and became foul; and Moses was angry with them. 21They gathered it morning by morning, every man as much as he should eat; but when the sun grew hot, it would melt.
22Now on the sixth day they gathered twice as much bread, two omers for each one. When all the leaders of the congregation came and told Moses, 23then he said to them, "This is what the Lord meant: Tomorrow is a sabbath observance, a holy sabbath to the Lord. Bake what you will bake and boil what you will boil, and all that is left over put aside to be kept until morning." 24So they put it aside until morning, as Moses had ordered, and it did not become foul nor was there any worm in it. 25Moses said, "Eat it today, for today is a sabbath to the Lord; today you will not find it in the field. 26Six days you shall gather it, but on the seventh day, the sabbath, there will be none."
27It came about on the seventh day that some of the people went out to gather, but they found none. 28Then the Lord said to Moses, "How long do you refuse to keep My commandments and My instructions? 29See, the Lord has given you the sabbath; therefore He gives you bread for two days on the sixth day. Remain every man in his place; let no man go out of his place on the seventh day." 30So the people rested on the seventh day.
31The house of Israel named it manna, and it was like coriander seed, white, and its taste was like wafers with honey. 32Then Moses said, "This is what the Lord has commanded, 'Let an omerful of it be kept throughout your generations, that they may see the bread that I fed you in the wilderness, when I brought you out of the land of Egypt."' 33Moses said to Aaron, "Take a jar and put an omerful of manna in it, and place it before the Lord to be kept throughout your generations." 34As the Lord commanded Moses, so Aaron placed it before the Testimony, to be kept. 35The sons of Israel ate the manna forty years, until they came to an inhabited land; they ate the manna until they came to the border of the land of Canaan. 36(Now an omer is a tenth of an ephah.)
A secondary, brief record is found in verses 4 through 9 of chapter 11 of the biblical book of Numbers.
4And the rabble who were among them had greedy desires; and also the sons of Israel wept again and said, "Who will give us meat to eat? 5We remember the fish which we used to eat free in Egypt, the cucumbers and the melons and the leeks and the onions and the garlic, 6 but now our appetite is gone. There is nothing at all to look at except this manna." 7Now the manna was like coriander seed, and its appearance like that of bdellium. 8The people would go about and gather it and grind it between two millstones or beat it in the mortar, and boil it in the pot and make cakes with it; and its taste was as the taste of cakes baked with oil. 9When the dew fell on the camp at night, the manna would fall with it.
I think the most curious and seemingly inexplicable property of manna found in these records is that it behaved differently on the sabbath than it did on other days of the week. It would spoil if left overnight preceding any of the six Israelite working days, but it would not spoil when left overnight preceding the sabbath.[5] I will call this Observation 1.
Observation 1: Manna would spoil if left overnight preceding any of the six weekly workdays, but it would not spoil when left overnight preceding the sabbath.What natural food substance behaves in such a curious fashion?
For anyone seeking a naturalistic explanation of manna, Observation 1 is a bit of a mind-bender. Unfortunately, it gets worse. Moses instructed that a jar of manna should be kept in perpetual storage in the tabernacle so that future generations might be able to see for themselves the manna which their ancestors had eaten in the desert.[6] Clearly, the manna kept in the jar in the tabernacle did not spoil when kept overnight on weekdays.
Observation 2: Manna kept in a jar in the tabernacle did not spoil overnight at all.
Perhaps the easiest explanation of these two observations is that the natural tendency of manna is to spoil overnight but that this natural tendency was supernaturally suspended 1) each night before a sabbath so the Israelites would not need to go hungry on the sabbath, and 2) while kept in the jar in the tabernacle for the benefit of future generations. A competing, similarly easy explanation is that the biblical record of manna is mostly mythological—that these are merely fictitious stories told to further the cause of Israelite religion. In point of fact, manna and its spoilage properties are neither supernatural nor mythological. Manna is a completely natural, real substance, and its spoilage properties have natural, real explanations, as subsequent pages will show.
This assertion may seem to some to be lacking in piety, so I need to pause and address this briefly before moving on.
The primary record of manna strongly supports the idea that God was actively involved in the preservation of manna for sabbath consumption.
See, the Lord has given you the sabbath; therefore He gives you bread for two days on the sixth day. (Exodus 16:29.)But notice that this does not say that God was supernaturally giving the Israelites "bread for two days on the sixth day." Nor is this implied, any more than it is implied that the giving of the sabbath, week by week, was supernatural. Recall that the pattern of six days of work followed by a day of rest is modeled on Creation Week.
Remember the sabbath day, to keep it holy. Six days you shall labor and do all your work, but the seventh day is a sabbath of the Lord your God; in it you shall not do any work, you, or your son, or your daughter, your male or your female servant or your cattle or your sojourner who stays with you. For in six days the Lord made the heavens and the earth, the sea and all that is in them, and rested on the seventh day; therefore the Lord blessed the sabbath day and made it holy. (Exodus 20:8–11.)God was clearly involved in establishing the pattern of six days of work followed by one day of rest. He did this in Creation Week. In the same way, God was clearly involved in establishing the natural preservation properties of manna. He, the Creator, is the one who established the entire natural order of the universe.
The biblical record of manna and its properties neither demands nor solicits a supernatural explanation, and neither should we. In fact, proper piety motivates us in the opposite direction. I explained this a quarter of a century ago, in connection with my quest back at that time for the physical cause of Noah's Flood, as follows.[7]
And this vital witness is still very much an object of my present effort to discover the physical cause of manna.Apologia
Should we look for a physical cause of the Flood? Isn't it somewhat impious to do so?
Yes, we should look for a physical cause of the Flood, and no, it is not impious to do so. In point of fact, given the present climate of rampant unbelief of the Bible among learned men and women world-wide, it is impious for Christians not to do so. Let me explain.
Genesis does not represent the Flood of Noah as a mythical saga. It presents it as sober, real-life history. So when a man or a woman concludes that Noah's Flood is mythological, they have automatically also concluded that Genesis is not a reliable historical witness. But if the Bible's historical witness in Genesis cannot be trusted, what basis is there for supposing that its historical witness in the Gospel of John can be trusted? If the Bible says the Flood happened when in fact it never did, should we trust the Bible when it says the Resurrection happened?
It will not help to try to separate the Old Testament from the New either, as if the Old could be mistaken but the New still sound, for the New Testament also treats the Flood as real-life history. It reads, "And just as it happened in the days of Noah, so it shall be also in the days of the Son of Man: they were eating, they were drinking, they were marrying, they were being given in marriage, until the day that Noah entered into the ark, and the flood came and destroyed them all."[8]
If Noah's Flood is myth, then the New Testament as well as the Old cannot be trusted. Indeed, in that case, even Christ cannot be trusted (may it never be!) for those are His words which we have just read above from Luke 17. He has then Himself mistaken a myth for real history. And how then can men and women be called to trust Him—to rest their eternal destiny in Him?
Is it not then the pious duty of every genuine Christian to exert themselves in whatever way they are able to show the historical factuality of Noah's Flood to the present, unbelieving generation?
As it turns out, there is no better way of doing this then by discovering the physical cause of the Flood. I will not try to explain why this is so here; it will, I hope, be abundantly clear by the time you have finished reading this article.
But I do not want my attempt to find the physical cause of the Flood to be misunderstood. I am a scientist and I will be bringing the tools of science to bear on this question as forcefully as I know how. But please do not suppose for even a moment that I am out to purge the Flood narrative of the supernatural by my effort to understand its physical cause. The Biblical narrative clearly portrays supernatural activity associated with the Flood, and I have no argument with the witness of the text. (Neither, for that matter, does true science, though many today, having imbibed deeply of the philosophical intoxicant called naturalism, would have us believe otherwise.) The timing of the Flood was clearly supernatural. The revelation of the impending judgment to Noah was clearly supernatural. Even the closing of the door of the ark was supernatural.[9] It would be folly, in my opinion, and a denial of the word of God to attempt to find a natural, physical cause underlying any of these things.
But the Flood narrative also involves much which is not supernatural. God could have supernaturally taken Noah, his family, and the animals out of the earth entirely, and returned them only after the Flood was all over if He had wanted to do so, but He chose rather that Noah should build a boat. God could have supernaturally zapped every unbelieving, unrepentant sinner in Noah's generation out of earthly existence to stand then and there before the judgment seat if He had wished to—leaving Noah and His family safely behind—but He chose rather to send a flood.
The natural and the supernatural are both there side by side. To deny either is to miss the truth. The supernatural is there that we might know that God is. The natural is there as a verifiable witness to what He has done.
It is this witness which is the object of my present effort to discover the physical cause of Noah's Flood.
Unfortunately, all of the purported explanations of manna as a natural substance which I have seen to the present time are woefully inadequate. To the logical mind, they serve only to materially assist the conclusion that manna must be either supernatural or mythological. As I much prefer the positive exercise of showing the truth over the negative exercise of exposing error, I will limit illustration to a single example:
In particular, there is a scale insect that feeds on tamarisk, the Tamarisk manna scale (Trabutina mannipara), the secretions of which are often considered to be the prime candidate for biblical manna.[10]According to this theory, parasitic insects of desert tamarisk trees excreted the manna reported in Exodus and Numbers.
The challenges presented to this "prime candidate" scale insect theory by the biblical record of manna appear to be insurmountable. Indeed, the challenge presented by Observation 1 alone appears to be fatal to the theory. Are the excretions of these insects found today to spoil and become foul and maggot infested overnight every day of the week except Friday nights? For that matter, are they ever found to spoil overnight at all? These questions are, discreetly, never even raised.
According to the biblical historical record, the Israelites numbered roughly two million men, women, and children during this journey.[11] Manna was a staple food of the Israelites while they were in the wilderness. Everybody got a fair share of it every day, "an omer apiece" according to Exodus 16:16. Thus, these insects needed to provide roughly two million gallons of manna per day. This, obviously, would require many millions of scale insects and millions of tamarisk trees. The manna kept coming as the Israelites moved from one location in the desert to another. So this theory seems to require that the desert was a virtual forest of tamarisk trees, all severely infested with scale insects, all busily excreting "manna" (except on the sabbath) so it would fall to the ground when the dew fell at night (Exodus 16:14, Numbers 11:9) for the people to gather it up each morning (Exodus 16:21). And this went on for forty years (Exodus 16:35) "until they came to the border of the land of Canaan" when the tamarisk trees and scale insects evidently suddenly ceased.
But I have been to the vast Negev desert where these Israelites camped, and I can attest first hand that tamarisk trees are few and far between in that desert. The wilderness where the Israelites camped presents a predominantly barren landscape, devoid of trees.
There is only one way this scale insect theory can be taken seriously as providing a "prime candidate" natural explanation for manna, and that is by simply ignoring most of what the biblical record about manna has to say.
But if this is to be the method, why bother? This is just unabashed cherry picking.
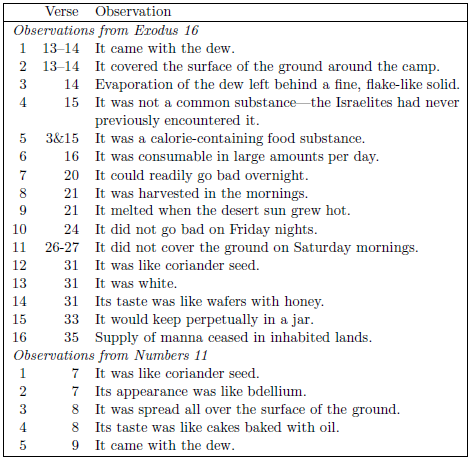
Biblical historical texts serve as a recorder (like a tape recorder) of historical events. What the recording provides are observational data. The observation that manna came with the dew (Numbers 11:9) is a piece of scientific data. The observation that there was no manna to be found on the ground on sabbath mornings (Exodus 16:27) is another piece of scientific data. The observation that "when the sun grew hot, it would melt" (Exodus 16:21) is another piece of scientific data. The biblical record of manna is loaded with scientific data (Table 3.1).
 |
Science operates on data to yield conclusions. But to get valid conclusions, the data must be treated objectively. Cherry picking the data breaks the most fundamental rules, ethical as well as methodological, of valid scientific enquiry.
Supposedly naturalistic explanations of manna which require that much of the biblical record be ignored do violence to the biblical historical record. And supposedly naturalistic explanations of manna which violate the fundamental rules of science do violence to science. Explanations like the scale insect theory are really neither biblical nor scientific.
So why bother? Ascribing manna to myth or to supernatural activity at least displays admirable scientific integrity, admitting up front that no explanation of manna as a natural substance has so far been found or appears even possible.
If we are to get at the truth about manna, rigorous adherence to a high view of the validity of the biblical historical record plus rigorous adherence to a high view of the validity of scientific principles and methods must be maintained, for both are from the same Creator. Anything less will only result in folly, as it has to the present time.
The seemingly contradictory biblical/scientific observations regarding the spoilage properties of manna—Observation 1 and Observation 2—are, in fact, keys which unlock the mystery of manna. They show immediately that manna spoils in certain environmental conditions but does not spoil in other environmental conditions. This observation is a property of many foods. If you leave your ice cream on the kitchen table, it will spoil overnight. If you leave it in the freezer, it will be fine. Tuna left in an open can on the counter will soon develop a bad smell, go moldy, and even grow maggots if flies are present. The same tuna will last for years in the unopened can.
The biblical record of manna teaches us that manna would not spoil if it was kept in a jar. Pottery jars of that ancient time often had lids, and the lids of pottery jars can be sealed tight if need be, using melted beeswax, for example. The Septuagint specifies a "golden pot," rather than "a jar," in Exodus 16:33, which is reflected in Hebrews 9:4. This would lend itself even more to long-term airtight storage. Evidently, to spoil, manna had to be left open to the air. It appears that manna, like tuna, can be preserved by keeping it from ambient air.
Now let us take another look at Observation 1.
Given the deduction that manna is sensitive to ambient air, Observation 1 says immediately that there was something different about the air in the Israelites' camps the night before the sabbath than on other nights of the week. Is there any reason why the air would have been different in their camps the night before the sabbath than it was the rest of the week?Observation 1: Manna would spoil if left overnight preceding any of the six weekly workdays, but it would not spoil when left overnight preceding the sabbath.
Yes, there most certainly is.
The Israelites behaved differently on the sabbath than on every other day of the week. They were forbidden to work on the sabbath. This regulation was taken very seriously. The biblical record teaches us that sabbath-breaking was a capital crime in ancient Israel. Moses had a man executed for gathering sticks for his fire on the sabbath, for example.[12]
The Israelites were pastoralists. They kept livestock: sheep,[13] goats,[14] and cattle.[15] We must not picture a few dozen animals accompanying the Israelites on their wilderness journey. There were millions of people and we must picture vast herds.
The livestock would have been led away from the camp to graze desert vegetation each morning,[16] and then herdsmen and livestock would have returned to camp each evening—and here is the critical point—except for evenings before sabbaths.
The herds could not be taken from the camp to graze desert vegetation sabbath mornings. This would be work—sabbath-breaking. It would be work for the herdsmen, and it would be work for the herds, and work was forbidden for both the herdsmen and the herds on the sabbath as we have already seen from Exodus 20:8–11. The sabbath was to be a day of rest. The herdsmen were to rest and the herds were to rest on the sabbath. But there would be insufficient vegetation for vast herds to graze on in the camp. Yet the livestock could not be left unfed on the sabbath. What was to be done?
The solution was for the herdsmen and the herds to remain out in the fields on the sabbath. They would not have returned to the camp the evening before the sabbath.
If you have ever visited a stockyard, you will know that high concentrations of animals significantly change ambient air. In Loda, we live just a few miles from one of the largest egg producing facilities in Illinois. The prevailing wind moves air from Loda off in the direction of this chicken facility. A few times each year, however, the wind switches direction for a brief spell, usually just prior to a storm. Whenever this happens, Loda air smells like chickens—lots of chickens. To the uninitiated, it stinks. But on those who have become accustomed to it, stockyard air generally has a different effect. In his book All Things Wise and Wonderful, James Herriot sketched the difference this way:
An old man was mucking out the byre and as the rich bovine smell drifted across, one of my companions wrinkled his nose. But I inhaled it like perfume.[17]
Archaeology teaches us that the Israelites' herds were kept on the outskirts of the camp at night.[18] Israelite camps no doubt settled into an optimal arrangement after the first few encampments. I picture a sea of varied tents surrounded by a vast stockyard each night (Figure 3.1). There would have been several advantages to this arrangement. It would have made a surprise attack on the camp more or less impossible, for example, and the dung produced by the livestock would, when dry, have provided a natural fuel for Israelite cooking fires, campfire fuel being otherwise difficult to procure for such a large population in a desert environment.
 |
Nighttime air in the camp would have been different from daytime air. Nighttime air would have been stockyard air—except for nights before sabbath. On those nights, the livestock were out in the fields rather than congregated together on the outskirts of the camp, and there would have been only normal desert air in the camp.[19]
We are no longer an agrarian society, so many readers might wonder why the herds should be brought back to the camp at all. In addition to the just-mentioned advantage of providing fuel for fires, the simplest reason is that the animals would have needed to be watered, and the camp would have been situated convenient to the watering hole. More complex reasons involve the daily use of these animals for many purposes by the Israelites living in the camps. The shepherds would surely have had animals for sale—for sacrifices or for eating, for example—and one could not expect potential buyers to hunt up the flock on the backside of the desert to make their purchase.
It might also be wondered today how it would have been possible to manage vast herds—to get large numbers of animals to go where they were supposed to go. This question is perhaps best answered by watching one or two Internet videos on sheep and shepherds. It is not uncommon for these to show a lone shepherd with a half dozen sheep dogs effortlessly herding thousands of sheep through a narrow gate, for example.
Observation 1 and Observation 2 are not contradictory. They find their explanation in 1) the laws of nature which God established at the outset should govern His created universe, and 2) the laws of conduct which God established at Sinai should govern His newly created nation.
With this much figured out, the manna mystery rapidly begins to unravel.
We may now reasonably conjecture, for example, that it was something in the stockyard air which caused manna to spoil.
And we may reasonably conjecture that the reason there was no manna to be found on the ground on sabbath mornings was because there were no livestock present surrounding the camp the previous night.
And this conjecture implies that the livestock were the fundamental source of the manna.
This is easily explicable scientifically today. Stockyard air is just normal air loaded with many different gases from volatile compounds emitted by animals—gases present in their breath, for example.
One volatile compound that livestock give off is acetic acid (the acid in vinegar). If you leave vinegar open on the table, it will slowly evaporate into the air causing the air to smell like vinegar.
The air of stockyards contains many such gases, creating a strong aroma distinct to each different type of stockyard animal.
We may reasonably conjecture that these volatile stockyard gases are the fundamental ingredients of manna.
Since the manna came with the dew, we may reasonably conjecture that certain of the stockyard gases with an affinity for water (the fundamental substance making up dew drops) concentrated in dew, where they reacted to form a substance which was left behind as a solid once the water had evaporated.
This solid residue was manna.
It is convenient to give this explanation of the origin of manna a name. I will call it the "stockyard gases theory of manna."
Stockyard Gases Theory of Manna: Manna is the solid residue remaining when the liquid is evaporated from dew exposed overnight to stockyard gases.
This theory immediately explains why the manna occurred wherever the Israelites camped in the desert. The manna would have come with the dew wherever the livestock were congregated overnight, and the livestock were congregated overnight around the camp, wherever in the desert the camp was located.
The stockyard gases theory of manna is a legitimate scientific theory. It does not cherry pick the data. It embraces all of the data. Because it embraces all of the many observations about manna recorded in Exodus 16 and Numbers 11, it is easily testable.
The theory itself readily provides a recipe for making manna.
Recipe for Making Manna: Expose dew to stockyard gases overnight. In the morning, evaporate the dew. The solid residue remaining is manna.
If the stockyard gases theory of manna is correct, then, when this recipe is followed, we must get from it a fine, flake-like substance (Exodus 16:14). This substance must be white (Exodus 16:31). This substance must melt in hot desert sunlight (Exodus 16:21). It must spoil overnight when in desert stockyard air and not spoil overnight in ordinary desert air (Exodus 16:20, 22-24). It must be foul when it spoils (Exodus 16:20). It must not be toxic (Exodus 16:35). It must have nutritional value (i.e., it must supply food calories) (Exodus 16:3).
This is not an exhaustive list, but it is sufficient to make the point that the theory is easily tested in numerous ways. It has essentially no chance of success if it is false. Falsifiability is the criterion of a legitimate scientific theory. According to this criterion, the stockyard gases theory of manna is a legitimate scientific theory for the origin of manna.
The first thing needed to make manna is knowledge of which gases are present in stockyard air, together with their relative abundances. Fortunately, this has been measured. I will use data from Table S5 (beef #1), Table S6 (beef #2), and Table S7 (sheep) of the Supplement of Emissions of volatile organic compounds (VOCs) from concentrated animal feeding operations (CAFOs): chemical compositions and separation of sources by Bin Yuan et al.[20]
These tables list emission ratios (ER) for 28 volatile organic[21] compounds (or, in a few cases, organic compound categories, such as "C7 acid") measured in the air coming from concentrated livestock operations.
The emission ratio for a gas is its concentration in air, measured in parts per trillion (ppt), divided by the concentration of ammonia (NH3) in the same air, measured in parts per billion (ppb). Emissions are normalized to the concentration of ammonia because ammonia is the most abundant gas in stockyard air signaling the presence of a concentration of animals.
The first line of each of these tables, for example, lists, in the first column, acetic acid, mentioned previously. The second column gives the total ER for acetic acid. The total ER includes the total of emissions from the animals, their waste, and their feed. The third column breaks out the portion of the total ER which is due to just the animals and their waste. This third column is the column of interest to the present work. The Israelites were not running a feedlot or feed yard. They led the livestock out to graze. They did not bring the feed to the livestock. So the stockyard gases present in the Israelite camps would have been due only to the animals and their waste.
We will be looking closely at a number of the 28 volatile organic compounds listed in each of these tables, but let us first take a look at ammonia, which we have just learned is the most abundant gas distinctive to stockyard air.
Ammonia takes us immediately into some chemistry of special interest to the present study. The stockyard gases theory of manna leads to the expectation of chemical reactions of stockyard gases in desert dew. Dew is water condensed from the air. As it turns out, ammonia, the most abundant stockyard gas, has a very high affinity for water. Looked at the other way around, water soaks up ammonia. More to the point in the present context, dew soaks up ammonia.
The affinity of ammonia for water is frequently demonstrated in chemistry classes by using what is called an "ammonia fountain," a somewhat confusing, shortened name for what is really a water fountain powered by the strong affinity of ammonia gas for water. Here is how it works.
An inverted flask is first filled with ammonia gas (not household liquid ammonia solution). Ammonia is lighter (i.e., less dense) than air, so it stays in the inverted flask. Next, a rubber stopper with a glass tube running through it is used to stopper the inverted flask, and the bottom end of the glass tube is immersed in water filling a large beaker (bigger than the flask). The glass tube functions like a straw in the water. To get the flask of ammonia to start sucking water up the straw and out of the beaker into the flask of ammonia, a small amount of water is added into the flask. This might be done using a small syringe full of water mounted in a second hole through the rubber stopper, for example. When the plunger of the syringe is depressed, adding a drop of water to the flask, the drop of water starts absorbing ammonia from the flask. As the ammonia is absorbed, a suction is created in the flask, pulling water up the straw. This water absorbs even more ammonia, causing an even bigger suction. As a result, water comes squirting into the flask from the straw, like a fountain. This continues until the flask is nearly full of water (the fullness depending on the purity of the ammonia gas in the flask). You can look up "ammonia fountain" on the Internet to see this demonstrated.
When ammonia (NH3) enters water, it can react with the water to form ammonium hydroxide (NH4OH). This compound can ionize in water to yield NH4+ and OH-. Though we have just begun, this already shows that there can be a lot more going on with a simple dewdrop than one might think. Already, with just ammonia present, the dewdrop contains not only H2O but also NH3, NH4OH, NH4+ and OH- in various concentrations.
Now water itself, you may recall, can ionize to produce H+ and OH-. If there is an excess of H+ in water (i.e., more H+ than OH-), then we say that the solution is acidic. If it is the other way around—more OH- than H+—then we say that the solution is basic.
Normally, dew will tend to be acidic. This is because carbon dioxide in the air dissolves in water to produce carbonic acid. Dew exposed to ammonia gas results in the dew gaining more OH-, as we have just seen. So dew exposed to ammonia will tend to become less acidic.
When I was in high school, we did a simple experiment in chemistry class one day. We mixed a base, sodium hydroxide (NaOH), with an acid, hydrochloric acid (HCl), in water in ceramic crucibles. Then we used our Bunsen burners to heat the solution and evaporate the water. Once the white residue had cooled, we were encouraged by the teacher to taste it. We were all very surprised to find, in this way, that we had just made table salt, sodium chloride (NaCl).
We can now see that manna may result from a similar process. The dew provides water. The ammonia from the stockyard air supplies a base. One or more of the small organic acids in stockyard air may then supply the acid for an acid–base reaction yielding solid manna once the water has evaporated.
The most abundant small acid, according to the three published tables cited previously, is acetic acid. This leads to the conjecture that manna may be just crystals of ammonium acetate.
Ammonium Acetate Hypothesis: Manna is crystalline ammonium acetate.
This hypothesis is supported by the fact that ammonium acetate is a white, crystalline substance, but it is rapidly falsified by two other observations. First, when one looks up the melting point of solid ammonium acetate, on Wikipedia.org, for example, one finds 235°F. This is much too high for manna. Manna, the biblical record tells us, melted when the sun grew hot. My experience in the Negev desert of Israel says that one should not be surprised to find this melting point temperature to be in excess of 100°F (Figure 4.1), but certainly not 235°F. In fact, the maximum temperature of ambient air in the Negev desert recorded in modern times at Beersheba (northern Negev) is 115°F and at Eilat (southern Negev) is 119°F.[22]
 |
The second falsifying observation is that ammonium acetate is very difficult, if not impossible, to isolate from aqueous solution by simple evaporation. The problem seems to be that ammonium acetate is both 1) somewhat volatile and 2) hygroscopic. It absorbs water and it doesn't like to let the absorbed water get away. When I used a hot air gun to blow 100–105°F air over an inverted watch glass (i.e., a small glass dish) containing an aqueous solution of ammonium acetate, a smell of ammonia mixed with vinegar resulted, and the solution remained liquid until the whole thing had evaporated. There was no remaining residue. Evidently, ammonium acetate is not manna.
Thus, the ammonium acetate hypothesis proves to be false.
The second most abundant small acid, according to the published tables, is propionic acid. Its presence in stockyard air gives rise to the conjecture that manna may be just crystals of ammonium propionate.
Ammonium Propionate Hypothesis: Manna is crystalline ammonium propionate.
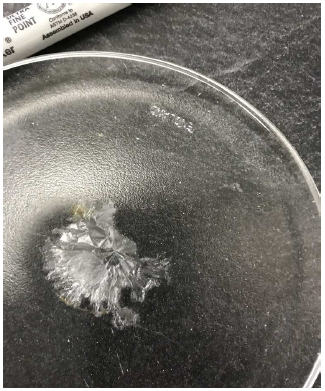
This hypothesis succeeds with the first three tests: 1) ammonium propionate is a white crystalline substance; 2) it has a melting point of 113°F, totally suitable to the biblical observation that manna melted when the sun grew hot; and 3) when I used a hot air gun to blow 100–105°F air over an inverted watch glass containing an aqueous solution of ammonium propionate, a solid crystalline residue remained when the liquid was gone (Figure 4.2).
 |
This theory also passes the toxicity test. Manna was used by the Israelites for forty years as a food item, so it must not be toxic. Ammonium propionate is, in fact, used today as a food additive, acting as a preservative against molds, fungi, and bacteria.[23] Clearly, it is not toxic to humans.
And it passes what I will call "the prolonged-preservation test." Its innate food-preservative property would explain why manna could be kept perpetually in a sealed jar without spoiling. Note that tuna will spoil if you take it from its sterile, vacuum-packed can and put it in a jar which is then sealed. Tuna has no innate food-preservative property. Microorganisms present in the air will get on the tuna as it is moved to the jar. These will grow on the tuna, causing it to spoil. To keep indefinitely, tuna has to be sterilized (and so does its container) and then kept sealed off from the air. Even ordinary bread will go moldy in a sealed container. But not so ammonium propionate. It acts against microorganisms, preventing their growth.
And it passes the nutrition test. Small organic acids supply about 3.1 food calories per gram, roughly three quarters as many food calories as do proteins and carbohydrates on a per weight basis.[24] A gallon of ammonium propionate per day—approximating the omer per day alloted to each Israelite—would yield about 3,000 food calories per day, assuming about the same packing density as settled snow (i.e., about three-fourths air). This is sufficient, by itself, to meet the daily calorie needs of an active adult male.
And it passes the spoilage test. Ammonium propionate is, like ammonium acetate, hygroscopic (though less so than ammonium acetate). In a high relative humidity environment, it will deliquesce, taking up moisture from the air to become, ultimately, a puddle. For long-term storage today, ammonium propionate must be kept in sealed containers to protect it from water vapor.[25] Stockyard air has elevated humidity because of water vapor exhaled by the animals and evaporated from their sweat and urine. The presence of dew each evening, recorded as part of the biblical description of manna, shows immediately that nighttime air in the Israelite camp, when the livestock were present, had high relative humidity, for dew forms only when relative humidity achieves 100%. So ammonium propionate can be expected to have turned from dry flakes to wet goo if left in an open container within the Israelite camp overnight whenever the livestock were present.
And it passes the foul test. Wet ammonium propionate will release ammonia gas and propionic acid vapor. The former has a "strong pungent odor,"[26] and the latter smells "pungent, rancid, unpleasant."[27]
Given the many successes of the stockyard gas theory of manna with ammonium propionate as manna itself, I was surprised by an unexpected difficulty in the lab. When I synthesized crystalline ammonium propionate, as previously described, I found it difficult to get the crystals completely dry. The crystals appeared somewhat translucent on the watch glass because of the moisture they retained, and when I scraped them off the watch glass, they were sticky on the spatula. I was eventually able to get the scraped-up crystals, which were sticking to the spatula, dry by using a heat lamp (Figure 4.3). The heat lamp was kept sufficiently distant to keep the temperature of the crystals slightly below their melting point.
 |
About a half hour of this drying procedure resulted in crystals which were no longer sticky and appeared to be white instead of translucent.
The idea that manna was sticky is absent from the biblical record. Manna seems to have been ready for collecting as soon as the dew had disappeared by evaporation. There is no mention of any required extra drying time or special handling necessary to prevent sticky manna.
It appears that, despite its many successes, the ammonium propionate hypothesis is also false. Manna is not simply crystalline ammonium propionate.
The third most abundant small acid, according to the published tables of stockyard gases, is butyric acid. Its presence in stockyard air gives rise to the conjecture that manna may be just crystals of ammonium butyrate.
Ammonium Butyrate Hypothesis: Manna is crystalline ammonium butyrate.
This hypothesis also enjoys many successes. For example, my experience working with butyric acid in the lab immediately showed it to be especially good at the foul test. It was easily detected at tiny concentrations in air, and it smelt really yucky. Its odor is described as: "unpleasant, similar to vomit or body odor."[28] Decomposing ammonium butyrate would surely have attracted flies in the Israelite camp.
But it, too, is false. It is falsified by the melting point test. The melting point of ammonium butyrate appears to be too high, exceeding 150°F.[29]
We could carry on in this way with other larger organic acids from stockyard air, but there seems to be little point. The amounts of these larger acids present in stockyard air are becoming vanishingly small—too small to supply the millions of gallons of manna needed each day. Already, the abundance of butyric acid in stockyard air is 50 times less than acetic acid, and the next larger acid, "C5 acid," pushes this down to 270 times less.
The stockyard theory of manna clearly exhibits many strengths, explaining most of the observations of manna found in Exodus and Numbers. Yet, in the end, it fails to give us a solid residue entirely suitable to manna. The stockyard theory of manna appears to be very much on the right track, but not yet the whole answer. An improved theory appears to be necessary.
As a general rule, when competing hypotheses all fail, as the three hypotheses of the previous chapter have done, it is a premise they all agree on which is false.
The stockyard gases hypotheses discussed previously all agree on three distinct premises. First, they all share the premise that manna is an ammonium salt. Second, they share the premise that manna is a pure crystalline substance. And third, they share the premise that all of the raw ingredients needed to make manna are present in stockyard air. All three of these premises are false.
The problem with the stockyard gases theory of manna is that it neglects the biblical observation that manna was exclusively a product of the desert. The biblical record is explicit that the manna ceased when the Israelites "came to an inhabited land" (Exodus 16:35). Evidently, one needs more than just dew and stockyard gases to get manna. One needs also the desert.
Explicit inclusion of the desert in the manufacture of manna alters the list of manna's potential ingredients and revolutionizes the conception of what manna was.
We are accustomed to seeing dewdrops on grass leaves. If you search "dew" on the Internet, the images which come up will be mostly, if not entirely, of dewdrops on leaves.
The three hypotheses of the previous chapter pictured manna as a pure crystalline substance, like a snowflake, left behind when the water had evaporated from a dewdrop. But the desert is short on leaves, and hence it is short on dewdrops. The desert is characterized by open ground—exposed soil. Dew does not sit in droplets on exposed soil the way it does on leaves. Like rain, dew soaks into soil. Now desert soils contain water-soluble inorganic salts. When dew soaks into desert soils, these inorganic soil salts provide additional potential manna ingredients.
Desert soils are uniquely rich in water-soluble inorganic salts such as sodium chloride, sodium sulfate, and magnesium sulfate. Such salts are flushed down below the root zone of cultivated soils in non-desert regions by the passage of rainwater through these soils. But in the desert, there is insufficient rain to accomplish this, and water-soluble salts accumulate relatively high up in the vertical soil profile.
When dew falls on such a soil, the water comprising the dew soaks into the soil where it has potential to dissolve soil salts. The dissolution and consequent mobilization of soil salts may seem to be a simple matter, but it is not. It plunges us immediately into aspects of soil science unfamiliar to most of the population but studied by those who work the soil and plant and harvest crops for a living. The important thing to know about from soil science in the present context is called the "CEC" (short for cation exchange capacity).
Cations (pronounced cat-ions) are just positively charged ions. Preceding chapters have made us familiar with ammonium (NH4+). Ammonium is an example of a cation.
Cations pair with anions (negatively charged ions, pronounced an-ions) because of the electrostatic attraction between opposite charges, to produce salts. When dissolved in water, salts ionize. That is, in water, salt molecules break apart into their constituent cations and anions. When the water is removed by evaporation, cation–anion pairs remain behind, producing the salt. For example, when dissolved in water, sodium chloride (table salt, NaCl) breaks apart into the cation Na+ and the anion Cl-. When the water is evaporated, solid crystals of NaCl remain behind.
Soils are porous. As a result, they absorb water. One might suppose that any water-soluble salt ion contained in the soil could be completely removed from the soil by flushing water through the soil, but this is not the case. Soil particles contain fixed negative charges on their surfaces. So the walls of a pore in the soil will be covered with fixed-in-place negative charges. These fixed charges can trap cations in the pore. Taking table salt as an example once again, Na+ ions can pair with wall-fixed negative charges, trapping the sodium cation in the pore, even when water is moving through the pore. This means that not all of a given water-soluble salt ion in a soil is freely available to be washed out of the soil by water.
Now imagine for a moment a soil which has such a large amount of salt in it that the fixed negative charges on the walls of the pores of the soil are overloaded with cations and cannot trap any more. Meanwhile, more salt is present in the soil as free anion–cation pairs. Dew falling on such a soil will produce a salts-laden aqueous solution in the pores of the soil. When the sun comes up, warming the land, it causes the water to evaporate from this salts-laden solution. Evaporation happens at the surface of the soil. As the surface water evaporates, its dissolved salts are left behind on the surface of the ground. Meanwhile, more salts-laden dew water is drawn up to the surface by capillary action. This water also evaporates, depositing yet more salts at the surface, and on it goes until the soil is dry once again.
The final result will be a layer of salts deposited on the surface of the desert soil. This layer may be vanishingly thin for light dew or soils with low concentrations of available salt, but it will be clearly visible for heavy dew on soils with high concentrations of available salt.
This phenomenon is called "efflorescence." The product of efflorescence—the surface layer of salts which efflorescence produces—is also (somewhat confusingly) called efflorescence.
Efflorescence is a common occurrence on masonry brickwork (Figure 5.1) where it is usually deemed an unsightly problem. Moisture entering the fabric of the bricks through their many pores dissolves available salts present in the bricks. When the water evaporates, a typically white efflorescence remains behind on the surface of the bricks.
 |
This leads to the conjecture that the "fine flake-like thing" found "on the surface of the wilderness" "fine as the hoarfrost on the ground" (Exodus 16:14) which the Israelites called "manna" was efflorescence.
But notice immediately that manna was not an efflorescence of just ordinary inorganic salts coming up out of the soil. Notice that efflorescence was unfamiliar to the Israelites. When they first saw manna, they asked "What is it?" Clearly, the Negev desert is not prone to producing efflorescence as a result of the action of dew on its inorganic salts alone. Manna had to have been an efflorescence of organic salts resulting from the passage into the Negev desert soil of organic acids from stockyard air dissolved in the dew. This is essential. Like table salt, inorganic soil salts yield no food calories. To provide manna with food calories, the salts comprising manna would need to have been primarily organic salts.
We may picture nighttime, moisture-laden, stockyard air producing a heavy dew soaking the Negev desert soil overnight. Ammonia and small organic acids present in the stockyard air readily dissolve in the dew, and the resultant solution soaks into the soil. The ammonia adds ammonium cations, and the acids add their anions (such as acetate, propionate, and butyrate) to the ions already present in the pores of the soil. The ammonium cations interact with soil salt cations trapped on the walls of the pores, freeing some of them. In the morning, when the sun rises and warms the land, evaporating the water, an efflorescence of organic salts is produced, made up of soil salt cations paired with anions from the organic acids in the stockyard air, covering a large acreage of ground between the herds and the Israelite camp. This flake-like efflorescence is easily harvested as a reasonably clean food item by first fanning or blowing it into piles.
The inclusion of Negev desert soil significantly modifies our conception of manna's potential ingredients, of how manna was formed, and of what manna was. A new theory, larger than just the stockyard gases theory, emerges. I will call this new theory "the stockyard gases efflorescence theory of manna."
Stockyard Gases Efflorescence Theory of Manna: Manna is an efflorescence from Negev desert soil when this soil has been exposed overnight to moisture-laden stockyard air.
Like the stockyard gases theory of manna, the stockyard gases efflorescence theory of manna is an easily testable, legitimate scientific theory. The method of testing it, once again, is to make candidate manna substances in the lab, drawing from the list of ingredients available in stockyard air and in the soils of the Negev desert, and then to evaluate these substances relative to what we know about manna from Exodus 16 and Numbers 11.
The recipe for making manna must now be expanded.
Recipe for making manna: Overnight, expose dew-drenched Negev desert soil to stockyard gases. In the morning, evaporate the dew. The efflorescence produced is manna.
The knowledge of stockyard gases called for by this recipe is in hand, but additional knowledge is now needed. We now need to know about the water-soluble inorganic salts present in Negev desert soils. Fortunately, this has also been measured. I will use data from Table 8.2.3-2 of The Soils of Israel in the following discussion.[30] This table gives the "Composition of salts in a saline loessial Serozem from the central Negev." The central Negev is of particular interest because we know from archaeology and the Bible that Mount Sinai, where the Israelites camped for a year and harvested manna the whole time, is located in the central Negev.[31] This table lists concentrations of soluble salt ions versus depth in this soil. No salts were directly measured. Rather, the positively charged ions and negatively charged ions comprising various soil salts were measured. The positive ions measured and presented in the table are sodium (Na+), potassium (K+), calcium (Ca++), and magnesium (Mg++). The negative ions are chloride (Cl-), bicarbonate (HCO3-), and sulfate (SO4–).
The list of interesting potential ionic ingredients furnished by stockyard air to the manna recipe had numbered just four: ammonium, acetate, propionate, and butyrate. The addition of the seven soil ions above expands the list to eleven. One can apportion eleven different ingredients in a very large number of different ways, giving rise to a very large number of candidate manna substances to be tested in the lab. Fortunately, not all of these soil ions turn out to be of interest.
Most of the soil cations are trapped. There is relatively little free salt in the surface layer of this soil. The total CEC for the upper 10 centimeters of soil is 18.6 cmol/kg. Meanwhile, free soluble salt cations totaled just 0.73 cmol/kg—a few percent relative to the trapped cations.
It is clear that the few percent of free salt in the soil did not contribute significantly to manna. The normal complete absence of efflorescence from central Negev soils makes this clear. Manna efflorescence only appeared whenever stockyard gases were present. Thus, most of the matter making up manna efflorescence had to have come from the stockyard air, not from the ground. This means that the free soil anions can be ignored. They will be present in manna at trace levels only. The anions present in manna solution will be dominantly the stockyard air anions acetate, propionate, and butyrate.
This leaves only the soil cations as potentially of interest to manna.
Trapped cations can be made available by exchange. This is what the cation exchange capacity (CEC) is all about. CEC is a measure of a soil's ability to trap cations and to exchange one cation for another. In the present context, cation exchange turns out to be the star role of the ammonia present in stockyard air. Plants need nitrogen atoms, of which ammonium ion is a source. So crop farmers, responsible for feeding us all, can often be seen injecting their soils with liquid anhydrous ammonia. When the ammonia reacts with water in the soil, ammonium results, filling up the CEC with ammonium cations plants can begin to feed on. The main expectation of the interaction of stockyard dew with Negev desert soil is addition of soil cations to the manna ion solution due to exchange with abundant ammonium cations.
A dominant characteristic of central Negev soils is that they tend to be highly saline. They contain a great deal of common table salt, sodium chloride (NaCl).
The highly saline soils of the Negev are concentrated in its central and southern parts.[32]This makes sodium abundant in these soils. The sodium cation, Na+, dominated the free salts of the upper 10 centimeters of the central Negev soil presently under consideration. It accounted for 89% of the cations measured in this surface soil layer. (Mg++ accounted for most of the remaining 11%, with K+ accounting for less than half a percent.) In the ancient Negev desert, some fraction of the relatively abundant ammonia in stockyard air would (in the form of ammonium in the dew-saturated soil) have wound up exchanging places with the abundant sodium trapped in the surface layer of soil, thereby freeing sodium ions. This particular cation exchange process would have contributed sodium cations to the manna solution.
Based on the relative abundance of magnesium (11%, mentioned above) in the small free ion pool measured in this soil, significant Mg++ would be expected to be present in the CEC. But because magnesium cation is doubly charged, it can be thought of as doubly trapped to the pore wall. This makes Mg++ more difficult than sodium, Na+, to free by exchange with ammonium, NH4+, so magnesium was likely to have been present in the manna solution in trace amounts only.
In general, ammonium is a "stronger" cation than sodium, but a "weaker" cation than the other cations of interest to this desert soil such as potassium, magnesium, and calcium.[33] "Stronger" cations may be expected readily to displace only "weaker" cations in cation exchange processes. Thus, when the manna solution evaporated at the surface, yielding the efflorescence mixture of organic salts called manna, it is the sodium salts of the organic acids found in stockyard air which may be expected to have dominated the efflorescence and given manna its distinctive properties.
As noted previously, acetic acid is the most abundant organic compound found in stockyard air, making acetate to be by far the most abundant anion in stockyard air dew. From a design perspective, with millions of Israelites to be fed, it makes sense that the most abundant stockyard air anion should be the main ingredient of manna.
When an aqueous solution of sodium ions and acetate ions is evaporated near room temperature, a translucent crystalline solid containing three water molecules per sodium acetate molecule in its crystal lattice results. This solid is called sodium acetate trihydrate. It is a hydrate, as its name shows, and the water it contains is called "water of hydration." My studies of sodium acetate trihydrate in the lab showed it to be a suitable main ingredient of manna in every way.
As just mentioned, sodium acetate trihydrate is a translucent crystal. This may seem immediately to disqualify it as a main ingredient of manna because manna, the biblical record explicitly states, was white. However, in warm dry air, sodium acetate trihydrate gives off some of its water of hydration, producing a whitest-of-whites coating of anhydrous sodium acetate. This release of water of hydration is also called efflorescence, further confusing the meaning of this over-taxed word. This kind of efflorescence—the spontaneous giving off of water molecules from a crystal to the air—may be thought of as the opposite of deliquescence, which is when a crystal absorbs water from the air eventually to dissolve and become a solution.
Sodium acetate trihydrate is about as well behaved in normal room air as is table salt. It is easily crystallized from aqueous solution by evaporation of the water. The resulting crystals are not sticky. I found that sodium acetate trihydrate crystals left on the counter in the lab overnight at roughly 50% relative humidity were unchanged the next morning.
To test spoilage of sodium acetate trihydrate in high humidity conditions, I placed a few crystals in a small beaker, and then I placed the beaker in a closed Mason jar with a small amount of liquid water covering the floor of the jar, producing a high relative humidity in the jar. By the next morning, the crystals had spoiled by turning to water droplets on the bottom of the beaker (Figure 6.1). Clearly, sodium acetate trihydrate, while efflorescent in warm dry air, is deliquescent in moist air, resulting in a solid which matches both the biblically-recorded color and the biblically-recorded overnight spoilage observations of manna.
 |
As a result of considerable experimentation over the many months which I spent on this research project, I learned that the melting point test single-handedly eliminated most manna candidates. In fact, ammonium propionate was the only exception I encountered. Melting points generally tended to be far too high, especially in the case of sodium salts. Surprisingly, sodium acetate trihydrate passed this test.
While anhydrous sodium acetate melts only at the high temperature of 615°F, the melting point of sodium acetate trihydrate is just 136°F. Pure sodium acetate trihydrate has this relatively low 136°F melting point temperature because of the water molecules it contains in its crystal lattice. When the temperature reaches 136°F, these water molecules are released, causing solid sodium acetate trihydrate to "melt" by dissolution in its own water.
While 136°F is an unusually low melting point for the organic and inorganic salts of potential interest to manna, it is still on the high side relative to record highs in the Negev desert. As discussed previously, record high outdoor air temperatures in the central Negev are expected to be a few degrees less than 120°F, so manna comprised entirely of sodium acetate trihydrate would not melt in outdoor air in the central Negev. But, once it had been gathered, manna would have been kept indoors, not outdoors, and achieving an elevated temperature inside a closed tent exposed to direct sunlight is not difficult. In addition, the melting points of mixtures are generally depressed relative to pure compounds. In the present case, solid manna will not be pure sodium acetate trihydrate. Rather, it will be a mixture of mainly sodium acetate trihydrate with anhydrous sodium acetate plus other salts from less abundant ions present in the manna solution, such as sodium propionate and sodium butyrate. So the unusually low melting point of pure sodium acetate trihydrate seemed, in fact, to be just about right.
I tested this using the following manna candidate recipe. To 10 ml of distilled water, I added 0.44 grams of sodium acetate trihydrate, 0.044 grams of sodium propionate, and 0.0070 grams of sodium butyrate.[34] Evaporation of the water from 0.465 ml of this solution on a watchglass using a stream of dried, 85°F air at 2 liters per minute yielded 0.022 grams of thoroughly dry solid in 90 minutes. When scraped off the watch glass, this gave a fine, flake-like, white solid, matching the biblical description of manna. The flakes were not at all sticky.
When I measured the melting point of these flakes (Figure 6.2), I found that they had clearly melted by the time the temperature had reached 129°F. This is only 10 or 11 Fahrenheit degrees above possible central Negev desert outdoor temperatures, an increase which is easily obtainable for a closed tent in direct sunlight. Thus, this manna candidate also passed the melting point test.
 |
I left the taste test to near the end of my investigations into manna for two reasons. First, basic strategy demands that the most definitive tests be given highest priority. Melting point, as already discussed, is highly definitive in the present case. In contrast, the taste test suffers from subjectivity—we humans vary in our perceptions of taste—making it less definitive.
Second, the biblical record of the taste of manna is complex, again making the result less definitive. Exodus 16:31 says "its taste was like wafers with honey" while Numbers 11:8 says "its taste was as the taste of cakes baked with oil." The Numbers observation is clouded by translational uncertainty. A marginal note, in the NASB being used here, informs the reader that "cakes baked with oil" is literally "juice of oil." Numbers then reads "its taste was as the taste of juice of oil."
In such a difficult textual situation, it seems best to set aside the details and focus on just the main ideas. But even this approach failed to improve definitiveness of the taste test. The main taste idea elicited by the Exodus observation, "its taste was like wafers with honey," is sweetness, while with Numbers the main taste idea appears to be oiliness. These are not the same things.
When I tasted half a teaspoon of sodium acetate trihydrate, I was surprised by the result. The initial sensation was of a brief, cool, mild sweetness reminiscent of artificial sweeteners. This was rapidly overwhelmed by a strong salty flavor which I don't know how to describe. It was unpleasant in the same sense that taking half a teaspoon of table salt would be. And then, most surprisingly, my mouth was left with a light aftertaste, or more precisely, a light aftersensation of oil/fat. The sweet versus oil complexity of the biblical observations immediately clarified. Sodium acetate trihydrate elicited both sweet and oily taste sensations.
The biblical description did not mention the overwhelming strong, salty flavor. Eventually, it occurred to me that this might be due to my use of an inappropriate sampling procedure resulting from my naivety with this substance. If we wish to determine whether white crystals in a container in the kitchen are salt or sugar, we do not sample half a teaspoon of each. Rather, we touch the top of our finger to our tongue to moisten it, then we touch the moistened finger to the crystals, and then we bring the few crystals which are stuck to the end of our finger back to our tongue for tasting.
When I tried this sampling procedure with sodium acetate trihydrate, the strong salty flavor was not present. In hindsight, this is clearly the right sampling procedure to use. Anyone familiar with manna would surely have used it. Manna salts are, just like table salt, sodium salts. If you take half a tablespoon of a sodium salt, you may expect to experience the unpleasantly briny taste of sodium overload, just as happens with table salt.
This realization raises the question of just how the Israelites managed to consume a gallon of manna per person per day, a question I will put off until the next chapter.
While sodium acetate trihydrate is a suitable main ingredient of manna, it will clearly not be the sole ingredient of manna.
When the emission ratios of the published measurements of stockyard air previously referred to (i.e., Table S5 (beef #1), Table S6 (beef #2), and Table S7 (sheep)) were averaged, acetic acid was found to comprise 86%, propionic acid 12%, and butyric acid 2% of the acids in this average stockyard air. This says that, in first approximation, for every 86 molecules of sodium acetate trihydrate in manna one might expect also to find in manna roughly 12 molecules of sodium propionate and roughly 2 molecules of sodium butyrate.
I was encouraged by the result of the sodium acetate trihydrate taste test to taste sodium propionate as well. I was surprised to find it more pleasant overall than sodium acetate trihydrate had been. I was also surprised to find that it was initially warm and sweet rather than cool and sweet.
With some trepidation I went on to sample sodium butyrate. Butyric acid smells awful, and I expected sodium butyrate to taste awful. I found that it tasted the best of the lot.
There will be varying trace levels of other salts in finished manna, as mentioned above, depending on details such as specific soil type at a given campsite, for example. Trace amounts of C5, C6 and higher stockyard air organic acids will be present, of course. Trace amounts of sodium chloride (i.e., table salt) from the ground are to be expected. Carbon dioxide, enriched in stockyard air via respiration, will likely result in trace levels of carbonate salts in manna. Even the "age" of finished manna gets involved in the discussion of trace levels of other salts, because ammonium salts will likely be present in trace amounts, and the general volatility of these salts would be expected to reduce their concentrations as manna aged.
This is not an exhaustive list of trace substances. Many substances may be present at trace levels, even dust from the desert floor. But none of these trace substances are expected to influence the properties of manna significantly.
A final potential ingredient which is of some importance to the potential properties of manna, impacting especially its taste, is sodium hydroxide (NaOH).
Recall that sodium is freed from the soil by cation exchange with ammonium and that the abundance of ammonia far exceeds the abundance of the organic acids in stockyard air. This means that, in principle, sodium has potential to exceed the amount needed to pair with organic acid anions in manna solution. This excess sodium could pair with hydroxide anions (OH-) from ionization of water molecules in manna solution. The resulting manna efflorescence would then contain sodium hydroxide.
The concentration of sodium in manna solution strongly impacts the taste of the finished manna. Too much sodium will yield a strongly basic manna with an unpleasant soapy taste from sodium hydroxide. Too little sodium will yield an acidic manna with a vinegar taste.
The biblical record leads us to expect manna to have neither an unpleasant soapy taste nor a vinegar taste. What is needed, to avoid these tastes, is just sufficient sodium to pair with the stockyard acids in manna solution and little more. Remarkably, when the equations governing the process are solved, this is found to happen automatically, as is shown in Appendix B.
It is now possible—with the help of a little mathematics needed to get the ratio of organic acids right (Appendix B)—to specify a recipe for making manna synthetically. This recipe ignores all trace substances which might be found in a native central Negev manna, leaving just the two principal ingredients comprising approximately 99.2% of the sodium salts in native Negev manna: 1) sodium acetate trihydrate and 2) sodium propionate. (The inclusion of sodium butyrate would add another 0.4%.)
Manna Synthesis: To 100 ml of distilled water in a container suitable for mixing (e.g., a 250-ml beaker), add 29 grams of sodium acetate trihydrate and 0.85 grams of sodium propionate. Mix these ingredients well, until completely dissolved. Weigh a glass pan or dish, such as the kind used to bake brownies, record its weight, and then pour the resulting manna solution out into this glass dish. Evaporate the water in a dehydrator at 125°F for 3 hours. The liquid will slowly disappear and, with about half an hour remaining, a dull crust will begin to appear. Once the crust has completed its formation across the entire solution, use a narrow spatula to scrape the damp product up off the glass, leaving the scraped up product in the brownie dish. Weigh the container plus manna clumps at this stage to calculate the weight of the product. Continue dehydration with frequent stirring until the weight of the product is 29 grams. The manna will continue to whiten during this final dehydration. Stop at 29 grams, because excessive dehydration will convert an excessive amount of sodium acetate trihydrate to anhydrous sodium acetate, evidenced by a low final product weight and the lack of a suitable melting point, ruining the batch. Finally, pass the final product through a mesh having eight wires per inch to achieve a size distribution suitable to the biblical "fine, flake-like" description. The product will keep indefinitely if stored in an air-tight jar.
The above recipe specifies a proportion of sodium propionate to sodium acetate trihydrate which has the greatest probability of matching native Negev desert manna based on available data. Nonetheless, variations in this ratio are obviously possible as 1) the emission ratios for propionic acid relative to acetic acid for the three ER tables underlying this recipe display variations of as much as 50%, 2) the composition of Israelite livestock would have differed from those used in the construction of these tables, and 3) the native Negev animal diet would have differed from the diets used in the construction of these tables. None of these factors is expected to make a significant difference to the properties of manna because of the overriding dominance of sodium acetate trihydrate in all cases.
The ingredients specified in the recipe have been chosen for their ready commercial availability and their safety in handling and transport. Nonetheless, ingredient substitutions are obviously possible. For example, sodium acetate trihydrate could be substituted by an equal molar amount of anhydrous sodium acetate or by an equal molar amount of sodium hydroxide plus an equal molar amount of acetic acid. As another example, sodium propionate could be substituted by an equal molar amount of sodium hydroxide plus an equal molar amount of propionic acid.
This recipe produces a pure white, crystalline solid product (Figure 6.3)
 |
which so obviously satisfies the biblical observations of manna in so many ways as to make absurd the notion that this product may not be manna. I am aware of only two residual biblical observations of manna needing further elaboration at this point: the observations 1) that "its appearance [was] like that of bdellium" in Numbers 11:7, and 2) that manna "became foul" in Exodus 16:20.
Numbers 11:7 says that the appearance of manna was like the appearance of bdellium.
Bdellium looks similar to hardened pine pitch (Figure 6.4). There is little similarity between its appearance and the appearance of manna.
 |
The Septuagint text has "hoarfrost" instead of "bdellium" in Numbers 11:7. It reads:[35]
And the manna is as coriander seed, and the appearance of it the appearance of hoarfrost.The similarity of manna to hoarfrost is mentioned also in Exodus 16:14.
When the layer of dew evaporated, behold, on the surface of the wilderness there was a fine flake-like thing, fine as the hoarfrost on the ground.The similarity of manufactured manna to hoarfrost is immediately obvious (Figure 6.5). This makes the leading explanation of this sole discrepancy between the scientific explanation of manna presented here and the ancient observations of manna recorded in the Masoretic text of Exodus and Numbers to be that "bdellium" results from a copy error of some sort with the Masoretic text in this instance.
 |
Theory, backed by lab experience, says that manna becoming foul will not be easily observed in the laboratory or in the kitchen today. Manna kept overnight in humid air will spoil (i.e., become a puddle), but it will not become foul. Manna exposed to high humidity spoils by becoming manna solution. Manna solution can always be converted back to solid manna by simple evaporation of the water once again. For manna to become foul, more than just high humidity is necessary. The overnight presence of stockyard gases is also necessary.
We are now aware that three stockyard gases are prime candidates for the foulness reported of spoiled manna: 1) ammonia, 2) propionic acid vapor, and especially 3) butyric acid vapor.
Finished manna, we now know, is lacking appreciable ammonium ions. Sodium ions have taken the place of ammonium ions. So wet manna will not emanate significant ammonia.
Wet manna will also not significantly emanate either propionic acid or butyric acid. To become odorous, an aqueous solution containing, for example, sodium butyrate (i.e., a solution containing CH3CH2CH2C(O)O- anions plus Na+ cations) must somehow convert butyrate to butyric acid. Butyrate cannot leave the solution and go into the air (to be detected as an odor by our noses) because its negative charge is attracted to the positive charge of sodium in the solution. Sodium butyrate (CH3CH2CH2C(O)ONa) cannot leave the solution because it is not volatile—it is a solid, not a liquid or a gas. Butyric acid (CH3CH2CH2C(O)OH) can leave the solution because it is both neutral (not charged) and volatile—it is a liquid which can evaporate into the air. But to turn butyrate into butyric acid in the solution, a source of H+ is needed. In theory, this might be supplied by splitting water into its ions, H+ plus OH-. But water is very reluctant to give up H+ to butyrate. So butyrate is more or less stuck in this solution and unable to get out to produce an odor.
Now, for an aqueous solution of ammonium butyrate, the case is quite different. Ammonium (NH4+) is relatively happy to give up H+ to butyrate. This turns the ammonium ion into ammonia gas (NH3) and butyrate into butyric acid. Since both are volatile and both are neutral, both may leave the solution—producing a stench. Similarly for propionic acid.
A bowl full of manna would have became foul when it spoiled overnight in stockyard air because the dew-drenched manna in the bowl would have concentrated the water-soluble substances ammonia, acetic acid, propionic acid, and butyric acid from stockyard air, just as the dew-drenched ground was doing all night. But, unlike the ground, the bowl would have provided no way for the ammonium to exchange with sodium. So in the morning, when the sun came up and warmed the land and the relative humidity dropped and the water began to evaporate from the manna in the bowl, ammonia gas and propionic acid vapor and butyric acid vapor would have emanated from the bowl—producing a stench.
This stench would surely have attracted flies and other insects, ultimately yielding what we call maggots—the "worms" recorded in Exodus.
The stockyard gases efflorescence theory of manna—that manna is an efflorescence from Negev desert soil when this soil has been exposed overnight to moisture-laden stockyard air—appears to be the true explanation of manna.
Manna was primarily about keeping the Israelites from starvation in the desert.
The problem of starvation would have arisen for the Israelites, not because of complete inaccessibility to food provisions, but because of the problem of preserving and transporting them while moving about in the desert.
Recall how, several centuries earlier, Joseph's brothers had come to Egypt to buy grain during Joseph's famine, and how the sacks of grain were loaded onto donkeys for the return trip home.[36] Ancient peoples were able to procure food by trade, and they were able to transport it long distances. But this was obviously an expensive way to eat which could not be sustained long term.
In addition, the necessity of transport of provisions from one desert campsite to the next prohibited normal means of storage at the individual household level. In the ancient world, household grain would normally be stored in very large pottery vessels. This would protect the grain from rodents, for example. But such vessels would be impractical for a mobile population, being too large and heavy to move about. The equivalent today would be to imagine taking your kitchen refrigerator along on a hiking expedition. The practical reality was that only relatively small quantities of commercially procured food provisions would have been possible. To prevent starvation, these meager provisions would need to have been supplemented with significant calories procured locally.
It might be supposed that the Israelites could have avoided starvation by eating their plenteous livestock. The Israelites knew better. They knew that this would work only until the livestock were all gone, and then complete catastrophe would follow.
The herds were essential to survival of the Israelites in the desert. The Israelite desert economy revolved around its herds. The herds were the Israelites' capital. Long-term financial strategy dictated back then, just as it does now, that the capital be preserved so it can be used continuously to produce income that one can live on.
The herds provided income to the Israelites by harvesting the desert. The income was in the form of food and fuel and raw materials for industry.
The herds provided food in the form of meat, of course, but this would have been carefully rationed. Overconsumption of meat would literally have been eating the capital. The herds would also have provided other food items, such as milk, yogurt, and cheese.
As previously mentioned, the herds also provided fuel for fires in the form of dried animal dung. This provided fires for cooking, for manufacturing (pottery vessels, for example), and for comfort.
And the herds provided raw materials for industry. Most obvious in this category would be wool for fabrication of cloth, needed for making tents and clothing. Another obvious commodity from the herds would be leather for footwear, straps, pouches, and more. All of this industry would have taken place not in great factories, but rather in individual households. Such manufactured goods would have provided items for trade with neighboring nations, enabling the Israelites to supplement their diets with figs, grains, pomegranates, honey, and other items mentioned in the narrative which they would not have been able to grow themselves during their desert wanderings.
The Israelites knew that they could not eat up their livestock. They needed to find local food calories elsewhere. Manna was the heaven-sent solution. It was an extremely frugal solution. It converted what were normally waste products—stockyard gases—into essential food calories free for the taking.
Exodus 16:16–18 records:
"This is what the Lord has commanded, 'Gather of it every man as much as he should eat; you shall take an omer apiece according to the number of persons each of you has in his tent.' " The sons of Israel did so, and some gathered much and some little. When they measured it with an omer, he who had gathered much had no excess, and he who had gathered little had no lack; every man gathered as much as he should eat.
This may seem to some to be saying that everybody miraculously got the same amount each day no matter how much or little they gathered, but I think it is intended to convey that manna was deemed to be communal property under God who sent it and that it was distributed equally to all Israelite citizens by executive order from Moses. The people were commanded to gather what they would need for eating each day, but it seems unlikely that everybody would have had the same luck gathering it every day. It seems likely that the gathering of the manna would have been carried out in an organized fashion, with tribes assigned to tracts of land and families assigned to specific plots within their tribe's tract. The yield of efflorescence would have been variable across the vast acreage being harvested, depending on such factors as distance from the flocks and direction of nighttime breezes, so not everybody would have had the same abundant harvest each day. But the harvest was pooled, perhaps at the tribal or even lower level, and, whether an individual had managed to gather much or little, each received a fixed distribution of manna each day.
At the point in my research when I first tasted sodium acetate trihydrate, my conception of how manna was likely used began to change. Specifically, the taste test of sodium acetate trihydrate dispelled the idea that manna was itself a finished food item like breads or crackers or cakes. It became clear that manna fit better in the category of a food ingredient, like table salt or flour, than it did in the category of a finished food. Food ingredients can be eaten by themselves, but they are generally not too palatable when eaten that way.
Maybe this should have been understood at the outset. Recall that Moses at one point, instructing the Israelites regarding the proper use of manna, said, "Bake what you will bake and boil what you will boil…" (Exodus 16:23). This implies that manna was being incorporated into different dishes prepared in various ways. And Numbers 11:8 tells us that "the people would go about and gather it and grind it between two millstones or beat it in the mortar, and boil it in the pot and make cakes with it…." There would, of course, be no reason to grind something which was already a "fine flake-like thing" and no reason to boil something which was obviously water soluble, coming from evaporation of dew, if the intention was to eat it on its own. Manna could clearly be eaten raw without any such preparation.
In hindsight, the text appears to be communicating that manna was incorporated into the recipe for breads and cakes at the step of mixing of the dry ingredients—i.e., at the step of grinding the grains into flour—and that it was incorporated into the recipe for soups and added into the soup together with other ingredients prior to boiling. Notice that the text is explicit that the people would "make cakes with it"—not "make cakes of it." This explains how the Israelites were able to consume a gallon of manna per day. They used their manna ration mainly as one of several food ingredients in a variety of recipes. It also explains where the Israelites obtained the many vitamins and other essential nutrients not present in manna which they would need to stay healthy.
Manna should be thought of as a food ingredient, like salt or flour, not as a finished food.
This suggests what may be the proper understanding of the biblical observation that manna "was like coriander seed" (Exodus 16:31).
At the point where this observation is recorded in the account of manna given in Exodus, the physical shape of manna has already been specified as a "fine flake-like thing" (Exodus 16:14), so this comparison to coriander seed does not seem to be specifying the physical shape of manna. In fact, coriander seeds are round and a bit oblong, not flake-like (Figure 7.1). That further clarification of the shape of manna was maybe intended seems at first a possibility. Specifically, when coriander seed is split, which is easy to do, each half may possibly be described as "a fine flake-like thing," as can be seen in Figure 7.1. The halves are shaped like little boats. This "like coriander seed" observation would then be saying that this was the dominant shape of desert-produced manna efflorescence flakes. But the manna record in Numbers seems also to exclude the shape of manna from the comparison to coriander seed. It says, "Now the manna was like coriander seed, and its appearance like that of bdellium" (Numbers 11:7). It thus seems clear that all aspects of appearance, including shape, were excluded from the comparison to coriander seed.
 |
In addition to shape, color and taste are also excluded from the comparison to coriander seed. The record in Exodus specifies both of these separately thus: "it was like coriander seed, white; and its taste was like wafers with honey" (Exodus 16:31). The record in Numbers also specifies taste separately: "and its taste was as the taste of cakes baked with oil" (Numbers 11:8).
If the comparison to coriander seed is not specifying shape, color, or taste, then what is it specifying?
It seems that it may be specifying the food category. Coriander seed may be eaten raw, but it is not very palatable in that form, its taste being rather strong. It finds its most widespread use as an ingredient in diverse dishes, functioning to add flavor, bulk, and nutrition. In this regard, manna is like coriander seed.
An essential requirement of manna is that an omer (roughly a gallon, or more accurately about 3.64 liters) of it per person per day had to be able to provide adequate food calories to prevent starvation.
The human body is made up of organic molecules, and it requires organic molecules for fuel to keep it going.
We now know that manna is comprised almost entirely of sodium acetate trihydrate and sodium propionate. Sodium cation is inorganic and provides no food calories. But acetate and propionate anions are organic, and they do provide food calories. As mentioned in an earlier chapter, small organic acids supply about 3.1 kilocalories of energy per gram.[37] I found that 60 ml of manna synthesized in the lab according to the synthetic manna recipe specified previously weighed 26 grams. Now sodium has an atomic weight of 23 grams per mole, sodium acetate trihydrate has a formula weight of 136 grams per mole, and water has a molecular weight of 18 grams per mole. So the acetate part makes up ((136 - 23 - 3 × 18)/136 × 100 =) 43% of the weight of sodium acetate trihydrate, and the propionate part makes up ((96 - 23)/96 × 100 =) 76% of the weight of sodium propionate. Small organic acids, therefore, comprise about ([ (43 /100) × 29 + (76/100) × 0.85 ] / (29 + 0.85) × 100 =) 44% of the weight of manna. At 3.1 kilocalories per gram of small organic acids, this comes to ((44/100) × 26 × 3.1 = ) 35 kilocalories (i.e., 35 Calories) per 60 ml of manna. Given that an omer is about 3.64 liters, the manna ration provided each Israelite with (35 × 3640/60 =) 2,100 Calories per day.
The reference daily intake (RDI) for food calories in the United States is 2,000 Calories for women and 2,600 Calories for men. Physically active individuals require more.
It is clear that an omer of manna per day was sufficient to keep the Israelites from starving.
It is, of course, remarkable that an omer of synthetic manna turns out to supply the right amount of food calories needed for the Israelites. This provides independent corroboration of the validity of the manna recipe which this new scientific analysis of the biblical record has deduced. But more than this, it once again strongly confirms the historicity of the manna narrative.
What did any ancient writer understand about food calories? These have been worked out by scientists only in the last two hundred years. And yet we find that an omer of manna provides a day's worth of food calories. Not two days' worth or two weeks' worth. And not two minutes' worth or two hours' worth.
For anybody making this story up, there is a broad range of wrong out-comes, and only a narrow range for the right outcome. And yet the narrative has the right outcome—thereby rendering untenable any other assessment of these ancient records than that they are simply, factually historical.
There is one final evidence that the manna recipe is right and that the pentateuchal records of God's dealings with the Israelites under Moses' leadership are simply factual.
While manna was a marvelous solution to the problem of starvation of the Israelites in the desert, according to the biblical narrative, it was intended as a short-term solution only.
The biblical narrative makes it clear that the Israelites were supposed to be resettled in their ancient homeland within a few years of the Exodus. Their passage through the desert from Egypt to Canaan was meant to be brief. But because of fear, the Israelite army rebelled and failed to deploy as planned. The result was a delay of forty years:
The Lord spoke to Moses and Aaron, saying, "How long shall I bear with this evil congregation who are grumbling against Me? I have heard the complaints of the sons of Israel, which they are making against Me. Say to them, 'As I live,' says the Lord, 'just as you have spoken in My hearing, so I will surely do to you; your corpses will fall in this wilderness, even all your numbered men, according to your complete number from twenty years old and upward, who have grumbled against Me. Surely you shall not come into the land in which I swore to settle you, except Caleb the son of Jephunneh and Joshua the son of Nun. Your children, however, whom you said would become a prey—I will bring them in, and they will know the land which you have rejected. But as for you, your corpses will fall in this wilderness. Your sons shall be shepherds for forty years in the wilderness, and they will suffer for your unfaithfulness, until your corpses lie in the wilderness. According to the number of days which you spied out the land, forty days, for every day you shall bear your guilt a year, even forty years, and you will know My opposition. I, the Lord, have spoken, surely this I will do to all this evil congregation who are gathered together against Me. In this wilderness they shall be destroyed, and there they will die.' " (Numbers 14:26–35.)
This judgment appears to have been largely enacted through the natural consequences of long-term excessive sodium intake resulting from manna consumption.
Manna, we now know, is made up of sodium salts. It would obviously have contributed a very large load of sodium to the Israelite diet, and long-term consumption of excess sodium is known to elevate risk of chronic disease.
A gallon of manna per day supplies a daily intake of about 390 grams of sodium. The March 2019 "Consensus Study Report" on Dietary Reference Intakes for Sodium and Potassium sponsored by The National Academies of Science, Engineering, and Medicine set the present adequate intake (AI) of sodium at 1,500 mg (i.e., 1.5 grams) for adults.[38] Thus, manna consumption by the Israelites resulted in sodium intake levels about 260 times larger than adequate. What health risks are associated with such large sodium intakes?
First, there is no health risk due to acute toxicity at these high levels. The Israelites died of many causes the forty years they spent in the wilderness, but being poisoned to death by too much sodium was not one of those causes. The "Consensus Study Report" established no upper tolerable intake levels (UL) on sodium intake due to "a lack of a toxicological indicator specific to excessive sodium intake." In other words, there is no evidence that sodium is acutely toxic at high levels.
There is evidence that restricting sodium in the diet can reduce risk of chronic disease.
There is sufficient evidence to characterize the relationship between sodium intake and risk of chronic disease. The CDRR [chronic disease risk reduction intake] is established using evidence of the beneficial effect of reducing sodium intake on cardiovascular disease risk, hypertension risk, systolic blood pressure, and diastolic blood pressure. Reduction of sodium intakes above the sodium CDRR is expected to reduce chronic disease risk within the apparently healthy population.[39]To reduce these heart-related risks, the "Consensus Study Report" recommended that sodium intake be reduced "if above 2,300 mg/day" (i.e., it recommended that sodium consumption be kept below 2.3 grams per day). This would limit manna intake to about one and a quarter tablespoons per day.
Before proceeding, it should be noted that the general population ignores this recommendation at present.
Most U.S. and Canadian population groups consume sodium above both the AI and CDRR levels.[40]What we are about to learn about prolonged excessive sodium intake from the Bible's record of manna consumption should encourage everybody to begin to take this recommendation more seriously.
The fact that for forty years millions of Israelites consumed manna way above the modern recommended limit adds significant new data to help characterize the relationship between sodium intake and risk of chronic disease. According to Numbers 14:22–35 and Deuteronomy 2:14, all of the fighting men, 20 years of age and older at the start of the Wilderness Wanderings, died within 40 years. This says that none of the 20-year-old cohort of men lived beyond 60 years of age, none of the 21-year-old cohort of men lived beyond 61 years of age, and so forth. This is anomalous. Moses recorded that, during his lifetime, the natural lifespan had dropped to 70 to 80 years, much as it is today.[41] Yet none of the fighting men in any cohort younger than 30 years of age lived past 70 years of age. The total count of warriors was roughly 600,000,[42] so there must have been something like 150,000 men in the total 20 to 30 age cohort. That they all failed to reach 70 years of age is remarkably anomalous. This clearly supports the modern evidence linking long-term excess sodium intake to risk of chronic heart-related disease.
Manna—a marvelous solution to the short-term problem of starvation—was never intended to be a long-term staple of the diet. Recall that the manna ceased as soon as the Israelites left the desert. Had the Israelite army fulfilled their divinely appointed task according to plan, excess sodium intake would have ceased then and there, and the result would have been no long-term excess sodium intake.
Modern nutritional science predicts that long-term dependence on manna as the source of necessary food calories will lead to accelerated cardiovascular death rates due to excess sodium intake. The ancient biblical record of the Israelites' 40-year-long journey from Egypt to Canaan confirms this prediction, verifying the manna recipe and demonstrating once again the historicity of the biblical record from which this recipe has been derived.
The melding of science and the Bible presented in this volume makes it possible for the first time to sketch the entire process which provided the Israelites with manna.
The vast herds bedded at night surrounding the Israelites' desert city-camps provided essential manna recipe ingredients into the air (Figure 9.1). The desert soil provided another vital ingredient from the ground.
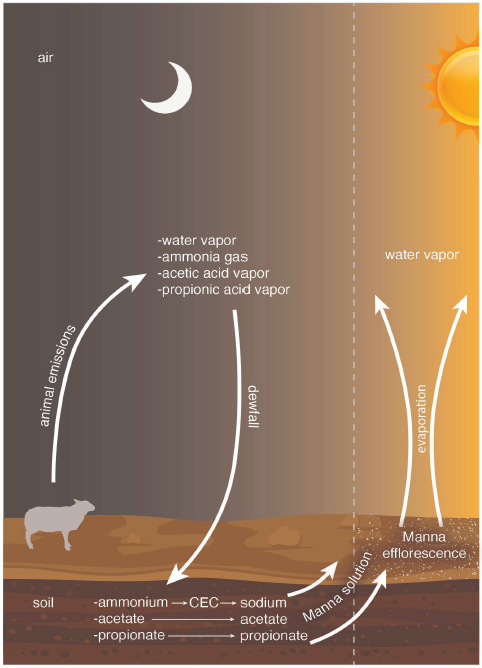 |
First and foremost from the herds was water vapor itself—otherwise a scarcity in the desert. This provided for a heavy dewfall each evening. The dew wet the soil, providing for interaction between stockyard air ions and soil ions.
Second in importance from the herds was ammonia. As the dew condensed from the stockyard air, wetting the surface of the soil, ammonia from the stockyard air was readily absorbed by it producing ammonium cations. This abundant cation not only helped create an aqueous solution which would readily absorb small organic acids from the stockyard air, but also, most importantly, it participated in cation exchange on the walls of soil pores, freeing abundant sodium ions for inclusion as a major manna ingredient.
The process of dew formation and absorption into the soil, together with manna ingredient stockyard gases, lasted all night, causing the wet zone of soil to extend slowly deeper, providing ready access to abundant soil sodium.
When the sun rose and began to warm the earth and the air, relative humidity began to drop. Water now began to evaporate from the surface of the soil back into the air. Any excess ammonia began to evaporate away. A mixture of minute crystals of principally sodium acetate trihydrate and sodium propionate, but also including trace amounts of other substances such as sodium butyrate, precipitated from solution, forming a fine, white, flake-like efflorescence layer covering the surface of the many acres of ground surrounding the Israelites' city-camp. The resultant solid was first fanned or otherwise blown into piles and then gathered up by the Israelites each morning. Any left behind would be recycled with the next dewfall.
Harvested manna efflorescence was hygroscopic. It would keep fine all day in the low relative humidity of the daytime desert air, but it would not keep overnight in the humidity-saturated, dew-producing stockyard air environment. It would take on water and water-soluble stockyard air gases. Ammonia gas and the small organic acids—most notably propionic acid and butyric acid—would slowly escape this solution once the water began to evaporate again the next morning, causing it to stink and attract flies and grow maggots. But on nights before the sabbath, when the herds were bedded out in the fields rather than surrounding the camp, ambient desert air in the camp was sufficiently dry overnight to prevent this spoilage.
With a more or less limitless supply of desert soil sodium and a perpetually renewable source of stockyard air, manna provided the millions of Israelites with necessary food calories for the entire forty years they spent in the desert. Desert soil efflorescence effectively converted gaseous animal waste products into a vital food item all the while the Israelites lived in the desert.
But there was a downside to manna. It overloaded the diet with sodium. This would not have been a problem had the Israelites left the desert and occupied their ancient homeland in a timely fashion. Their failure to do so condemned them to long-term excess dietary sodium, which resulted in the premature deaths of an entire generation of males.
When they finally left the desert and entered lands having sufficient rainfall to support agriculture, the manna ceased, agricultural soils being necessarily low in sodium ions.
Who masterminded this ingenious scheme, by which millions of slaves were freed from bondage to the then most powerful nation on earth and returned to their ancestral home?
Moses was surely a major player. His genius for leadership cannot be disputed. He wrote the oldest extant book of laws governing a nation. And via his code of laws, he quelled the never-ending spiral downwards of arbitrary reprisals for real and imagined wrongs, enabling millions of individuals to live in close proximity as part of a reasonably safe, tolerable, mutually beneficial and united social entity. As imitation is the sincerest form of flattery, the many codes of law produced by subsequent rulers of other nations—such as the Code of Hammurabi—even down to the present day with the ceaseless activities of legislative bodies worldwide, heap universal acclaim upon Moses' inventive leadership genius.
But we must go back before Moses to get this right. Moses could arrange the organization and leadership of the emergent nation of Israel. He could arrange to record the unfolding development of the nation as it happened day to day before his eyes, and he could arrange to record all of the wonders and miracles which happened along the way. But Moses could not arrange that acetic acid should be the most abundant volatile organic compound in stockyard air. He could not arrange that the cation exchange complex of the uppermost soil layer of central Negev desert soil be dominated by sodium. He could not arrange that stockyard air sport abundant ammonia gas. He could not arrange that ammonia gas should readily dissolve in water. He could not arrange that ammonia gas should react with water to produce ammonium. He could not arrange that soil should have a cation exchange capacity. He could not arrange that ammonium cation should act to free sodium cation from the soil. And yet all of this, and so very much more besides, had to be arranged just so, or the emergent nation of freed slaves would have starved to death in the wilderness despite Moses' leadership genius.
And so it seems, in fact, that we must give the credit for the masterful plan exhibited by the Exodus of the Israelites from Egypt and their subsequent passage through the desert back to their ancestral home to the One who predicted to Abraham, some 450 years previous to Moses, that this is what would take place.[43]
13Then God said to Abram, "Know for certain that your descendants will be strangers in a land that is not theirs, where they will be enslaved and oppressed for four hundred years. 14But I will also judge the nation whom they will serve, and afterward they will come out with many possessions. 15As for you, you shall go to your fathers in peace; you will be buried at a good old age. 16Then in the fourth generation they will return here, for the wrongdoing of the Amorite is not yet complete."
The ancient Israelites called this One "Jehovah." We call Him "God." Other tongues and nations know Him by many different names. He calls Himself "I AM" (Exodus 3:13–14), a name declaring timeless self-existence. He is universally recognizable, regardless of choice of proper noun, by His attribute of being the Creator of the universe.
 |
A question of importance to the present book is the relative aridity of the central Negev desert at the time of the Exodus, 2450 B.C. The amount of water needed daily for two million people plus accompanying vast herds of livestock would clearly not be small. In addition, vast herds would require a sufficiently dense cover of desert vegetation to graze upon each day, and the density of vegetation would clearly be dependent on the relative aridity of the desert.
The central Negev seems too sparsely vegetated to support vast herds today. When I visited the region in 2000, I saw one or two instances where it appeared a dozen animals were being grazed, but nobody was grazing vast herds. This is not surprising. Dryland agriculture becomes rapidly more difficult as annual precipitation falls below 10 inches per year, and average annual rainfall at Yeroham, Israel, where the biblical Mount Sinai is located, is just 6.9 inches today.[44] The cutoff for successful dryland agriculture using just natural rainfall (no irrigation) is probably not much below 9 inches per year.
Successful dryland farming is possible with as little as 230 millimetres (9 in) of precipitation a year;…[45]It thus appears that the Negev desert must have been less arid at the time of the Exodus than it is today.
That this was, in fact, the case is shown by modern measurements giving the elevation of the surface of the Dead Sea for the past roughly 8,000 years (Figure A.1).
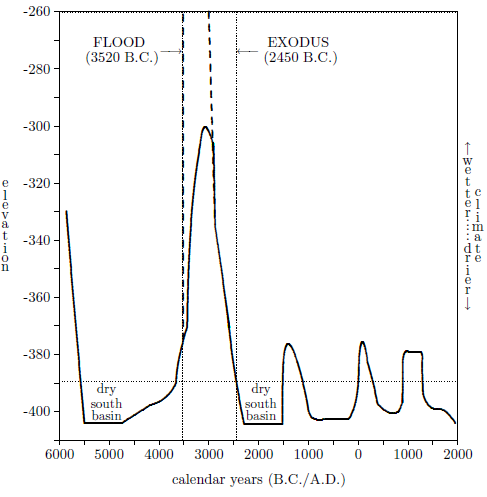 |
I have previously explained how the Dead Sea shows relative aridity in the region as follows:[46]
Since the Dead Sea has no outlet, the level of the Dead Sea depends only upon the rate at which water enters it through runoff and the rate at which water leaves it through evaporation. When the climate of the catchment basin (including the Jordan Valley) is arid, less water enters the Dead Sea (because there is less rainfall) and more water leaves the Dead Sea through evaporation. Under these conditions the level [or elevation] of the Dead Sea falls. When the climate of the catchment basin is moist, runoff into the Dead Sea increases and evaporation decreases so the level of the Dead Sea rises.
I corrected the elevation curve published by the original researchers, who had neglected the impact of Noah's Flood on the Dead Sea, in my book Noah's Flood Happened 3520 B.C.[47] The two dashed lines in Figure A.1 were added at that time. These two dashed lines, when extended upward, intersect at +60.5 meters, far above the graphed region, yielding a peak approximating the probable actual elevation of the Dead Sea surface due to filling of the Dead Sea depression by Noah's Flood.
The coming of the Flood inundated the northern hemisphere of the earth with ocean water for several months in 3520 B.C. This subsequently reduced aridity for some time, as might be expected. It took roughly a thousand years for the climate of Israel to return to pre-Flood aridity according to Figure A.1.
At the time of the Exodus (vertical dotted line at 2450 B.C.) the elevation of the Dead Sea (horizontal dotted line) was significantly higher than in modern times, corroborating the expectation from the biblical historical narrative that the region was less arid back at that time than it is today.
Given water vapor, carbon dioxide, ammonia, acetic acid, propionic acid, and butyric acid as the gases of interest to manna production, calculate the relative proportions of ingredients in Negev desert manna resulting from these gases in stockyard air.
Seinfeld and Pandis assume room temperature (298°K or 25°C) for all calculations. This should reasonably approximate the actual Negev desert night air temperature for dewfall.
Water vapor is added into stockyard air via respiration and by evaporation of sweat and urine. It is the most abundant of the stockyard air gases.
The ionization equation for water is:
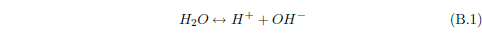
The equilibrium equation is:
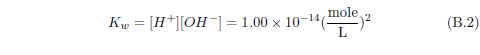
Carbon dioxide is the second most abundant stockyard air gas. It is a product of respiration and also of decomposition of animal manures. Carbon dioxide is of potential interest to manna production because of its large abundance and because it is water soluble, yielding an acid.
Carbon dioxide gas dissolves in water to produce carbonic acid, H2CO3.
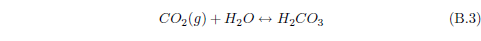
The corresponding equilibrium equation is
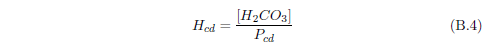
The value of Hcd is given by Sander as 3.3× 10-2 mole/L-atm.
Pre-industrial ambient CO2(g) levels were roughly 280 ppm. Stockyard air would have CO2 levels elevated above ambient. Assuming double the ambient level will probably be accurate to within a factor of 2. This sets
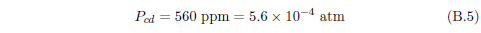
This yields the concentration of H2CO3 in Israelite Negev desert stockyard dew.
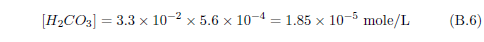
Ionization of carbonic acid in water yields
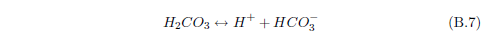
with equilibrium equation
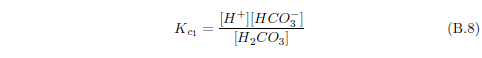
Kc1 is given as 4.30×10-7 mole/L in Seinfeld and Pandis, Table 6.4.
Carbonate (HCO3-) ionizes in water to produce H+ and CO32-
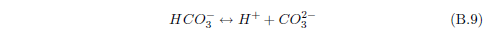
with equilibrium equation
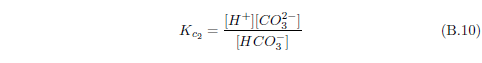
The value of Kc2 is given as 4.70×10-11 mole/L in Seinfeld and Pandis, Table 6.4.
Animal manures are the major source of ammonia in stockyard air. Ammonia dissolves in water yielding ammonium hydroxide, NH4OH,
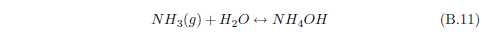
with equilibrium equation
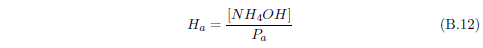
The value of Ha is given by Sander as 6.0× 101 mole/L-atm.
This yields the concentration of NH4OH in Israelite Negev desert stockyard dew.
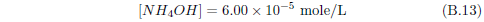
It is difficult to gauge what the concentration of ammonia would have been in the Negev desert stockyard air. It is expected to have been high because of the large number of animals surrounding the campsite. Bin Yuan et al. recorded levels in excess of 1 ppm for cattle, showing that such a level is possible. The unpleasant pungent smell of ammonia begins to be detectable only near 5 ppm. Safety concerns arise around 25 ppm. I will assume 1 ppm. This seems more likely to be too low than too high. This affects only the absolute concentrations of organic acids in manna solution, not their relative concentrations.
Ionization of ammonium hydroxide in solution yields
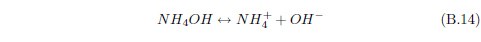
with equilibrium equation
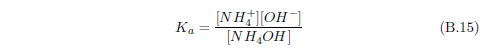
The value of Ka is given as 1.70×10-5 mole/L on page 353 of Seinfeld and Pandis.
Acetic acid is the most abundant volatile organic acid in stockyard air. It is water soluble
with equilibrium equation
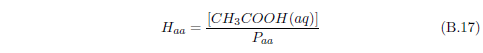
The value of Haa is given by Sander as 4.1× 103 mole/L-atm.
The average ER for acetic acid from Bin Yuan et al. is 29.25 yielding 29,250 ppt as its concentration in Israelite Negev stockyard air.
This yields the concentration of CH3COOH(aq) in Israelite Negev desert stockyard dew.
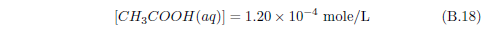
Ionization of acetic acid in solution yields
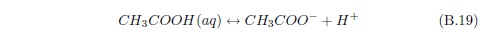
with equilibrium equation
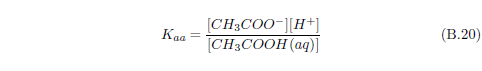
The value of Kaa is given as 1.70×10-5 mole/L on page 359 of Seinfeld and Pandis.
Propionic acid dissolves in water
with equilibrium equation
The value of Hpa is given by Sander as 1.5× 103 mole/L-atm.
The average ER for propionic acid from Bin Yuan et al. is 4.21 yielding 4,210 ppt as its concentration in Israelite Negev stockyard air.
This yields the concentration of CH3CH2COOH(aq) in Israelite Negev desert stockyard dew.
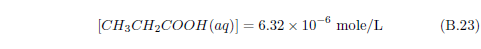
Ionization of propionic acid in solution yields
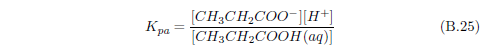
with equilibrium equation
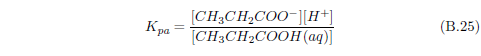
The value of Kpa is given as 1.3×10-5 mole/L in the table of acid ionization constants at www.chm.uri.edu.
Butyric acid dissolves in water
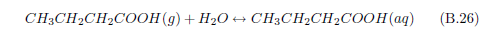
with equilibrium equation
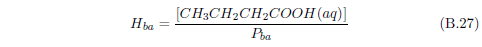
The value of Hba is given by Sander as 9.8× 102 mole/L-atm.
The average ER for butyric acid from Bin Yuan et al. is 0.58 yielding 580 ppt as its concentration in Israelite Negev stockyard air.
This yields the concentration of CH3CH2CH2COOH(aq) in Israelite Negev desert stockyard dew.
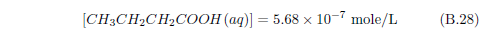
Ionization of butyric acid in solution yields
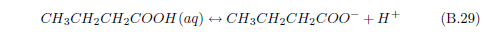
with equilibrium equation
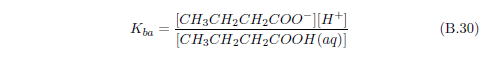
The value of Kba is given as 1.5×10-5 mole/L in the table of acid ionization constants at www.chm.uri.edu.
The foregoing sections yield a set of simultaneous equations which, when combined with the charge neutrality equation and then solved, yield ion concentrations in stockyard dew at the surface of the ground.
The charge neutrality equation is:
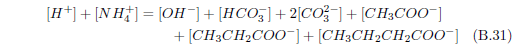
This equation together with the seven ionization equilibrium equations yields a set of eight equations in eight unknowns. This set of equations may be easily solved using a spreadsheet.[48] When this is done, the result is:
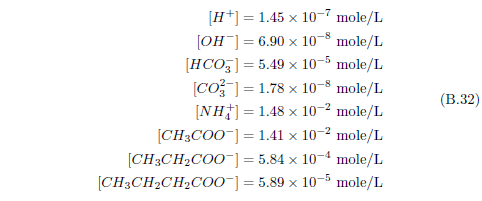
Notice that the pH of the solution at this point is (-log([H+] =) 6.8, so it is slightly acidic. Notice also that ammonium cation and acetate anion dominate this solution.
Once this dew solution enters the ground, the solution is closed off from the air. This allows the concentrations of the dissolved gases to begin to change for the first time. This introduces five new unknowns, which requires five new equations to be able to solve for the 13 concentrations of interest. These new equations are all conservation equations.
The total of the carbon dioxide species must now be conserved.
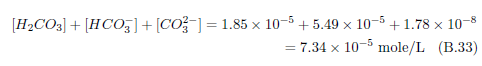
The total of the ammonia species plus sodium cation in solution must be conserved.
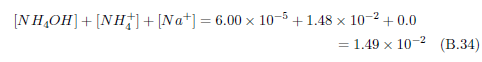
Similarly, the organic acids must be conserved.
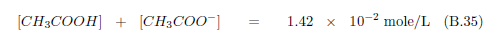
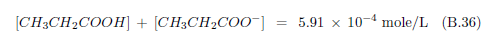
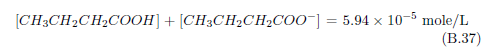
Meanwhile, ammonium cations begin to exchange with sodium cations to yield manna solution. The introduction of sodium cation changes the charge neutrality equation. It now becomes:
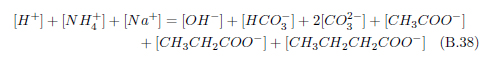
The resulting set of thirteen equations in fourteen unknowns can be solved by first specifying the sodium cation concentration. The concentration chosen will depend on how far the exchange of ammonium with sodium is allowed to proceed. This is expected to go to near completion because of the small diameters of soil pores and the hours of reaction time available each night. Solving the complete exchange case by choosing [Na+] = 1.48×10-2 mole/L, the maximum allowed by equation B.34, results in the following concentrations for manna solution:
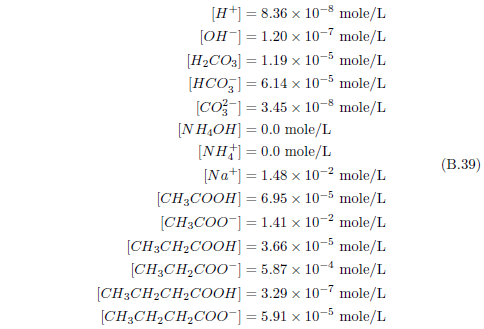
The pH is now 7.08, which is very slightly basic—the taste will be neither soap nor vinegar.
The manna product is clearly dominated by sodium and acetate. Propionate is present at just 4.2% of acetate. The next most abundant molecule is acetic acid. It is present at just 0.5% of acetate. Because it is volatile, much of it would be expected to evaporate off of manna flakes together with excess water each morning. All other species, including butyrate and bicarbonate, are present in this manna solution at less than 0.5% of acetate.
The important result is that manna flakes will be made up almost entirely of sodium acetate trihydrate with a small admixture of sodium propionate. A manna synthesis recipe which contains 4.2 moles of sodium propionate per 100 moles of sodium acetate trihydrate is expected to simulate closely the native central Negev manna first eaten by the Israelites 2450 B.C.
^ Gerald E. Aardsma, The Exodus Happened 2450 B.C. (Loda, IL: Aardsma Research and Publishing, 2008). www.BiblicalChronologist.org.
^ Gerald E. Aardsma, Aging: Cause and Cure (Loda, IL: Aardsma Research and Publishing, 2017). Gerald E. Aardsma, Addendum to Aging: Cause and Cure (Loda, IL: Aardsma Research and Publishing, July 26, 2019). Gerald E. Aardsma and Matthew P. Aardsma, Aging: Cause and Cure, 2nd ed. (Loda, IL: Aardsma Research and Publishing, 2021). Gerald E. Aardsma, Aging: Cause and Cure, 3rd ed. (Loda, IL: Aardsma Research and Publishing, in press). www.BiblicalChronologist.org.
^ See, for example, www.biblicalchronologist.org/store/obtain_vitamins.php.
^ en.wikipedia.org/wiki/Omer_(unit) (accessed December 24, 2022). This Wikipedia article begins with a thorough explanation of the derivation of the conversion of the ancient omer unit to the modern liter unit based on historical sources. This derivation finds 1 omer = 3.64 liters = 0.96 U.S. gallons. The article then mentions that the Jewish Study Bible (2014) uses 1 omer = 2.3 liters = 0.61 U.S. gallons. Unfortunately, the article does not comment on the 37% difference between these two conversion factors. As the more modern derivation seems to me more likely to be correct, I have adopted it in the present book.
^ Exodus 16:19–20, 23–24.
^ Exodus 16:32–34.
^ Gerald E. Aardsma, "The Cause of Noah's Flood," The Biblical Chronologist 3.5 (September/October 1997): 1–2. www.BiblicalChronologist.org.
^ Luke 17:26,27.
^ Genesis 7:16.
^ en.wikipedia.org/wiki/Manna (accessed June 1, 2022).
^ Gerald E. Aardsma, The Exodus Happened 2450 B.C. (Loda, IL: Aardsma Research and Publishing, 2008), 47. www.BiblicalChronologist.org.
^ Numbers 15:32–36.
^ For example, Exodus 29:38.
^ For example, Leviticus 16:5.
^ For example, Exodus 29:10.
^ Desert vegetation would have been more abundant at the time of the Exodus than it is today because the region was less arid back at that time (Appendix A).
Desert shrubs seem to be the major item for grazing in modern times. Searching "Negev desert shrubs" images on the Internet quickly reveals the nature of this food source and aspects of its distribution today. But there may also have been a substantial presence of grasses at the time of the Exodus due to the less arid climate back then.
Vegetation is much more plentiful in the wadi beds than it is in the open plains, so they would have been obvious targets for the shepherds.
Vegetation is seasonal. Rainfall peaks today in January and February. The months of June through September are dry. It appears that, in some places, springtime vegetation can result even today in something approaching what we would call a field.
Regardless, it seems certain that the vast herds must eventually have more or less denuded the desert surrounding wherever the Israelites camped, requiring frequent changes of campsite. This denuding of the desert seems possibly to be reflected in Numbers 22:4, "Now this horde [the Israelites] will lick up all that is around us, as the ox licks up the grass of the field."
^ James Herriot, All Things Wise and Wonderful, (New York: St. Martin's Press, 1977), 216.
^ E. D. Oren and Y. Yekutieli, "North Sinai During the MB I Period—Pastoral Nomadism and Sedentary Settlement," Eretz-Israel 21 (1990): 6–22. (English translation provided by Marganit Weinberger-Rotman.)
^ The absence of the flocks surrounding the camp on the night before the sabbath would have resulted in a subsequent shortage of animal dung fuel for collection for fires. This seems likely to be the practical reason underlying Moses' edict "You shall not kindle a fire in any of your dwellings on the sabbath day" (Exodus 35:3).
^ Supplement of Atmospheric Chemistry and Physics, 17, 4945–4956, 2017.
^ Organic compounds are compounds containing one or more carbon atoms. The biochemistry of living organisms is based on organic compounds.
^ en.wikipedia.org/wiki/Negev (accessed September 9, 2022).
^ www.amfoodchem.com/product/ammonium-propionate/ (accessed July 21, 2022).
^ en.wikipedia.org/wiki/Food_energy (accessed June 21, 2022).
^ www.amfoodchem.com/product/ammonium-propionate/ (accessed July 21, 2022).
^ en.wikipedia.org/wiki/Ammonia (accessed July 21, 2022).
^ en.wikipedia.org/wiki/Propionic_acid (accessed July 21, 2022).
^ en.wikipedia.org/wiki/Butyric_acid (accessed August 13, 2022).
^ commonchemistry.cas.org/detail?cas_rn=14287-04-8 (accessed August 13, 2022).
^ Arieh Singer, The Soils of Israel (New York: Springer, 2007), 246.
^ Gerald E. Aardsma, "Yeroham: the True Mount Sinai," The Biblical Chronologist 6.4 (July/August 2000): 1–11. www.BiblicalChronologist.org.
^ Arieh Singer, The Soils of Israel (New York: Springer, 2007), 243.
^ See, for example, the cation series 10.8a of L.R. Snyder and J.J. Kirkland, Introduction to Modern Liquid Chromatography, 2nd edition (New York: John Wiley & Sons, INC., 1979), 422. An online source, showing a similar cation series, may be found at mwclearreflections.com/brining-and-regeneration-part-i/ (accessed 2022/12/01) under the figure heading "Relative Affinities of Various Cations for Cation Exchange Resin."
^ All of these chemicals were purchased from Millipore-Sigma.
^ Sir Lancelot C. L. Brenton, The Septuagint Version: Greek and English (Grand Rapids: Zondervan Publishing House, 1970), 189.
^ Genesis 42–44.
^ en.wikipedia.org/wiki/Food_energy (accessed June 21, 2022).
^ nap.nationalacademies.org/resource/25353/030519DRISodiumPotassium.pdf (accessed December 4, 2022).
^ nap.nationalacademies.org/resource/25353/030519DRISodiumPotassium.pdf (accessed December 4, 2022).
^ nap.nationalacademies.org/resource/25353/030519DRISodiumPotassium.pdf (accessed December 4, 2022).
^ Gerald E. Aardsma, "Psalm 90 Is About Loss of Human Longevity Following Noah's Flood," The Biblical Chronologist 11.01 (October 14, 2021): 1–4. www.BiblicalChronologist.org.
^ Numbers 1:46.
^ Genesis 15:13–16.
^ championtraveler.com/dates/best-time-to-visit-yeruham-il/ (accessed 2023/01/06).
^ en.wikipedia.org/wiki/Dryland_farming (accessed 2023/01/06).
^ Gerald E. Aardsma, "Mount Sodom Confirms Missing Millennium," The Biblical Chronologist 1.1 (January/February 1995): 1–4. www.BiblicalChronologist.org.
^ Gerald E. Aardsma, Noah's Flood Happened 3520 B.C. (Loda, IL: Aardsma Research and Publishing, 2015). www.BiblicalChronologist.org.
^ In Excel, for example, the Goal Seek tool can be used to zero the net charge, via the charge neutrality equation above, by varying the hydrogen ion concentration.
 |