
 | |
| Volume 10, Number 11 | June 22, 2020 |
This issue and next are devoted to putting on public record two New Dietary Ingredient Notifications (NDINs) recently mailed to the FDA for Dr. Aardsma's Anti-Aging Vitamins dietary supplement. One NDIN is for vitamin MePA and the other is for vitamin MePiA. My PhD scientist son, Matthew, coauthored these NDINs with me.
Making these notifications available here allows you to see what has been communicated to the FDA about these new vitamins. These NDINs should be especially of interest to anyone who is wondering about the safety of Dr. Aardsma's Anti-Aging Vitamins. The main purpose of NDINs appears to be to cause the manufacturer or distributer of a dietary supplement containing a new dietary ingredient to give due consideration to the safety for consumption of the new dietary ingredient. Each NDIN must specify:[1]
The history of use or other evidence of safety establishing that the dietary ingredient, when used under the conditions recommended or suggested in the labeling of the dietary supplement, will reasonably be expected to be safe, including any citation to published articles or other evidence that is the basis on which the distributor or manufacturer of the dietary supplement that contains the new dietary ingredient has concluded that the new dietary supplement will reasonably be expected to be safe.
Normally, an NDIN author restricts to toxicology data alone. Normally, FDA does not want information about efficacy (i.e., does the dietary supplement actually do any good) in an NDIN because FDA has, by law, no jurisdiction over efficacy of dietary supplements. These MePA and MePiA NDINs are exceptions to this normal rule. I first submitted an NDIN to the FDA for MePA in 2017, focusing on toxicology and leaving efficacy pretty much aside. This, unfortunately, resulted in FDA refusing to address the toxicological safety or otherwise of Dr. Aardsma's Vitamin MePA Dietary Supplement, saying that:[2]
Although your notification states that your product, "MePA" is a vitamin, it does not provide adequate information to support the basis on which you make this conclusion.
I have previously pointed out to the FDA that their stance with this earlier NDIN is out of line both legally and ethically.[3] I have no legal obligation to prove to the FDA's satisfaction that a claimed new vitamin is indeed a new vitamin. If the FDA wants to dispute the categorization of MePA as a new vitamin, the onus is on FDA to prove that MePA is, in fact, not a vitamin. This does not put much of a burden on the FDA. The definition of a vitamin is so restrictive that, to the present time, out of the tens of millions of chemical compounds known to science, only 13 are classified as vitamins in modern nutritional textbooks. If MePA is not properly classified as a vitamin, it should be a trivial task to prove it.
Much more disturbing has been the FDA's insensitivity to the moral/ethical liabilities necessarily incurred by impeding commercial distribution of this new vitamin. The consequence of any impediment is unavoidably to aid and abet human suffering on a massive scale.
I have no desire to fight with the FDA over any of this. My goal is to alleviate human suffering and save as many lives as possible, as quickly as possible. To this end, the present NDINs do not argue over legality or ethics, but rather attempt to aid the FDA with its apparent need of additional evidence that these new vitamins should be classified as vitamins. To do so, it has been necessary to discuss not just toxicology but also efficacy in these present NDINs. The only way to show that a substance is a vitamin is to show that it is efficacious against its corresponding deficiency syndrome.
The standard scientific way to show that MePA and MePiA are efficacious against aging would be to run a clinical trial with half of the participants receiving a placebo and half receiving the vitamins. This, unfortunately, is both impractical and severely unethical. Given all that we know about aging at the present time, the clinical trial would need to begin with young children and follow them up through adulthood. When, after 80 or 90 years, all of the control group had died of aging and none of the treated group had died of aging, then the clinical trial would have demonstrated that the new vitamins do, indeed, appear to be efficacious. Unfortunately, several generations of the global population would have died of aging while waiting on the result from this clinical trial. Clearly, ethics and practicality demand a different approach.
Fortunately, one does not have to run a clinical trial to see that MePA and MePiA are vitamins. Adequate evidence is available in other ways, as the NDIN for MePA published below, and the NDIN for MePiA published in the next issue show.
Gerald E. Aardsma
Aardsma Research & Publishing
412 N Mulberry Street
Loda, IL 60948
methylphosphonic acid (CAS Number 993-13-5; Linear Formula CH3P(O)(OH)2; Molecular Weight 96.02) [abbreviated as MePA below]
The dietary supplement will be sold in a plastic dropper bottle containing not more than 20 mL of a 50:50 v/v solution of 125 mg methylphosphinic acid (MePiA) per liter water mixed with 125 mg MePA per liter water to yield 62.5 mg of MePiA and 62.5 mg of MePA per liter of dietary supplement.
Sec. 190.6(b)(3)(i) Level:
2.5 μg MePA per drop (the volume of a drop of water from the dropper bottle is nominally 40 μL.)
Sec. 190.6(b)(3)(ii) Conditions of Use:
The anticipated bottle label, with its conditions of use, is shown below

The anticipated contents of the "Fact Sheet" which will accompany each bottle of supplement, also displaying conditions of use, are shown below.
This fact sheet is intended to help you use Dr. Aardsma's Anti-Aging Vitamins dietary supplement intelligently and beneficially.
Dr. Aardsma (a Ph.D. research scientist, not an M.D.) has discovered that human aging is a vitamin deficiency disease of two closely related long-lost vitamins: methylphosphinic acid (MePiA) and methylphosphonic acid (MePA). According to Dr. Aardsma's research findings, these vitamins were naturally present in drinking water thousands of years ago. A global catastrophe (known from the Bible as Noah's Flood) halted their natural production five and a half thousand years ago. As a result, they are no longer present in drinking water. There appears to be no other natural source of these two vitamins.
MePiA and MePA have only recently been discovered. Research into these vitamins is still underway to answer such basic questions as the optimal daily intake. Dr. Aardsma's Anti-Aging Vitamins dietary supplement is designed to conveniently provide the best present estimate of an optimal daily intake of MePiA and MePA.
According to Dr. Aardsma's research:
Modern human aging is a syndrome of three underlying diseases: (1) Aging 0: congenital MePA deficiency disease, (2) Aging 1: congenital MePiA deficiency disease, and (3) Aging 2: a mitochondrial disease induced by Aging 1, probably subsequent to age 10.
The absence of MePiA and MePA in human diets results in the well-known, ultimately fatal, aging syndrome.
Because these anti-aging vitamins are no longer present in drinking water, 100% of the global population presently suffers from aging.
Before Noah's Flood, when these vitamins were naturally present in drinking water, people were living in excess of 900 years.
Restoration of MePiA and MePA to human diets has potential to improve health by curing Aging 0 and Aging 1.
Restoration of MePiA and MePA to human diets has potential to prevent Aging 2 in young individuals (probably less than age 11) and thereby greatly increase their life expectancy.
Whether restoration of MePiA and MePA to human diets can ameliorate or cure Aging 2 is, so far, unknown.
Before Noah's Flood, everybody who drank water took MePiA and MePA without ever being aware of it. This would have been everybody except nursing infants. (It seems probable that nursing infants obtained these vitamins through the mother's breast milk, though this has yet to be demonstrated.)
This suggests the simple rule that everyone other than nursing infants should take MePiA and MePA. However, things have gotten considerably more complicated in regard to health and medicine than they were thousands of years ago, so this rule needs to be applied intelligently and cautiously.
For example, today some individuals are organ recipients (e.g., kidney, bone marrow, heart, etc). These individuals require artificial suppression of their immune systems for their transplanted organs to be accepted by their bodies. The anti-aging vitamins appear to revitalize the immune system, which could conceivably lead to rejection of transplanted organs. So far, there has been no research done on this. Thus, individuals having transplanted organs need informed, professional medical guidance before beginning to take vitamins MePiA and MePA, and they need careful medical supervision once they begin taking these vitamins.
You should take vitamins MePiA and MePA daily for the rest of your life.
Your body needs to be supplied with all of the vitamins continuously, on a daily basis. A balanced diet will supply all of the known vitamins except these two anti-aging vitamins. Unlike the naturally occurring situation for thousands of years in the past, MePiA and MePA are no longer naturally available in any known food or drink. It is essential that you continue to supplement your diet with these two vitamins on a daily basis to provide your body with the MePiA and MePA it needs for normal maintenance, growth, and development.
Active ingredients:
methylphosphinic acid, 2.5 microgram per drop
methylphosphonic acid, 2.5 microgram per drop
Take the number of drops of Dr. Aardsma's Anti-Aging Vitamins dietary supplement indicated in the following table in a glass of water daily.
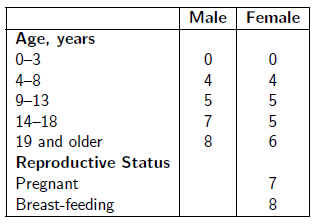
See www.biblicalchronologist.org/RDI.php for elaboration of this table and for the most current daily intake recommendations.
Ask your medical caregiver before use if you are the recipient of an organ transplant.
Do not give Dr. Aardsma's Anti-Aging Vitamins dietary supplement to breastfeeding children.
When taken as directed, Dr. Aardsma's Anti-Aging Vitamins dietary supplement appears to be free of negative side effects.
Keep refrigerated. Keep the bottle tightly capped to minimize loss of water due to evaporation. Shelf life exceeds two months under these conditions.
As with any vitamin supplement, it is a good idea to discuss with your medical caregiver the inclusion of Dr. Aardsma's Anti-Aging Vitamins dietary supplement in your diet.
To avoid running out of Dr. Aardsma's Anti-Aging Vitamins dietary supplement, keep an extra bottle on hand at all times.
You can keep up with the latest anti-aging vitamin research developments at www.BiblicalChronologist.org.
The FDA regulates dietary supplements under the authority of the Dietary Supplement and Education Act (DSHEA) amendment of the Federal Food, Drug, and Cosmetic Act (FFDCA). DSHEA specifically includes vitamins as dietary ingredients of dietary supplements, including them in the "Dietary Supplements" food category and excluding them from the drug category. MePA and MePiA are both vitamins according to the normal definition of "vitamin" including the FDA definition of "vitamin." Thus, Dr. Aardsma's Anti-Aging Vitamins is a dietary supplement, not a drug.
Dr. Aardsma's Anti-Aging Vitamins is a dietary supplement specifically designed to treat, cure (to the extent possible), and prevent the human aging syndrome. At present, 100% of the global human population is afflicted with this syndrome.
The Food and Drug Administration (FDA) does not approve dietary supplements. You must decide for yourself whether a dietary supplement is of benefit to you and whether you should take it or not. Your medical doctor or other health professional can help you with this decision. To help both you and your health professional, we make every effort to publish our research as open-access content at www.BiblicalChronologist.org as quickly as possible and to be as transparent as possible. Our goal is to use these newly discovered vitamins to improve health and to save as many lives as possible from the now unnecessary aging syndrome.
prepared by Gerald Aardsma, Ph.D.
last updated June 10, 2020
Vitamin.
This categorization results from decades of research into the cause of human aging by Gerald E. Aardsma, PhD, briefly summarized below. MePA and MePiA have only recently emerged from this research as a vitamin duo responsible for modern human aging.
FDA does not define "vitamin" merely as a list of the thirteen traditional vitamins. This is necessary because the thirteen traditional vitamins were discovered over a protracted period of time and there has never been any scientific reason to suppose that the discovery process has ended or that these thirteen vitamins exhaust the vitamin category.
Casimir Funk, whom many regard as the "father of vitamin therapy"[4] observed that:
The study of degenerative pathological changes of old age… may well belong to a future chapter of vitamin research.[5]Given that Funk would have been intimately familiar with the displayed phenotypes and physiological symptoms of human vitamin deficiency cases as well as animals experimentally deficient in specific vitamins, such as shown in Figure 1,
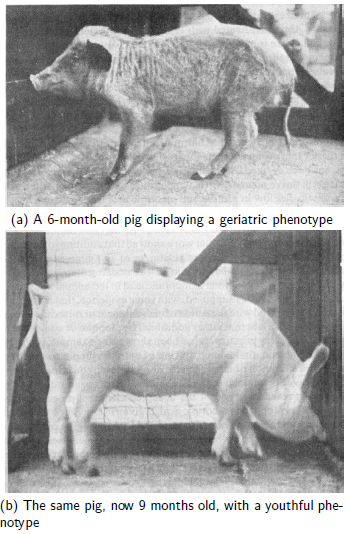 |
this statement by him is not surprising. MePiA and MePA appear to be properly classified as a duo of anti-aging vitamins, in fulfillment of Funk's conjecture. Aardsma's research finds that modern human aging is a syndrome of three diseases:
Aging 0: congenital vitamin MePA deficiency disease,
Aging 1: congenital vitamin MePiA deficiency disease, and
Aging 2: a mitochondrial disease induced by Aging 1.
MePA, a water-soluble weak acid, behaves in a way which is characteristic of water-soluble vitamins. Compare it to dietary supplementation with the water-soluble weak acid nicotinic acid, for example. Clinically advanced pellagra is rare in the U.S. today, but before the discovery of nicotinic acid in the later half of the 1930s, pellagra was common in the southern states where corn was a major dietary stable. Following is a description from back at that time[6] of the effects on pellagra patients of treatment with nicotinic acid.
A comprehensive report has been made by Spies, Bean, and Ashe, based on observations at the Cincinnati General Hospital, and the Hillman Hospital, Birmingham, Alabama, on the nicotinic acid treatment of hundreds of cases of classic pellagra. It is stated that:Relief of diverse symptoms with no hint of any negative side effects due to treatment is characteristic of the cure of a deficiency disease by a water-soluble vitamin. This same behaviour is seen with MePA. Following is a summary of experience gained to date with volunteers taking MePA in μg amounts per day, deliberately imitating the report on the nicotinic acid treatment of pellagra cases quoted above, to show the similarity."The administration of adequate amounts of nicotinic acid or one of its compounds is followed by the disappearance of many symptoms of the disease. Within 24 to 72 hours [1 to 3 days], the fiery redness and swelling of the tongue, gums, mouth, throat, and vagina subside, and the associated Vincent's infection disappears. Within 24 to 72 hours, nausea and vomiting cease, the increased salivation decreases, and bowel movements become normal. Abdominal distention, pain and discomfort disappear and, in most cases, the desire for food returns. The acute, fiery red erythematous [reddening of the skin, usually in patches] dermal lesions, in which the epithelium [thin tissue forming the outer layer of a body's surface] is intact, blanch within 48 hours after the administration of nicotinic acid, but where the continuity of the skin is broken and the lesions are moist, ulcerated, dry or pigmented, there seems to be no specific benefit. Perhaps the most dramatic response of the pellagrin to nicotinic acid therapy is the disappearance of the acute mental symptoms. These symptoms, varying from slight confusion to delirium and mania, disappear rapidly, often over night. The maniacal patients become calm and the confused patients, mentally clear. After therapy they become readjusted, and often have excellent insight and memory of their actions, ideas and surroundings during the psychotic period. Apathy and lassitude give way to interest."
The administration of adequate amounts of methylphosphonic acid is followed by the disappearance of many symptoms of the disease of aging. Within a few weeks to a few months, the sleep disorders characteristic of aging subside: there is less trouble getting to sleep (i.e., reduced insomnia), sleep is deeper and more refreshing, and less sleep is needed. Associated fatigue is reduced or disappears. The rate of wound healing is remarkably increased, and accompanying inflammation and pain is decreased. The incidence of headaches and migraines is reduced. Within a few weeks to a few months, diseases which have taken hold because of agedness, such as heart failure, cancers, and autoimmune disease, may begin to be slowed, reversed, or cured. Numerous skin disorders disappear: skin becomes more moist and supple; chronic skin infections begin to clear up within a month after the administration of methylphosphonic acid; aging spots begin slowly to fade. Perhaps the most dramatic response of the elderly to methylphosphonic acid therapy is the disappearance of chronic mental symptoms. These symptoms, varying from "brain fog" to depression and anxiety, disappear rapidly, sometimes within the first week. The depressed become more happy, the anxious, more calm, and the "brain fogged," mentally clear. Apathy and lassitude give way to interest and creativity.Experience gained to date with MePA demonstrates the relief of diverse symptoms with no hint of any negative side effects which is characteristic of the cure of a water-soluble vitamin deficiency disease.
MePA fulfills classical definitions, as well as FDA's definition,[7] of a vitamin as follows:
an organic substance
– MePA (linear formula CH3P(O)(OH)2) is an organic substance.
that is a minor component of foods,
– MePA is missing from all natural dietary sources today but was present in minor amounts (μg per liter) in drinking water for millenia in the distant past up until the cessation of its natural environmental production c.a. 2500 B.C.[8]
is essential for normal physiological functions (e.g., maintenance, growth, development),
– MePA is essential for maintenance. Modern individuals supplementing their diet with synthetic MePA report improved health and physiological function similar to what was normal for them as younger individuals. These reports are in diverse physiological areas such as a heart-failure patient on the verge of a heart transplant experiencing a very unexpected return of heart function, a dramatic reduction in benign prostate hyperplasia symptoms, improved sleep quality and energy levels, improved wound healing, improved mental function and health, decreases in asthma and allergies, fewer number of illnesses such as the common cold, and other areas of improved health.[9] Many of these findings are self-reported by individuals supplementing their diet with MePA and remain to be evaluated in formal research studies. However, the repeated reporting of statistically unlikely events by these individuals leaves random chance as a poor explanation for the observed benefits. Instead, the self-reported evidence indicates that when MePA is present in the human diet, physiological maintenance of the body is improved, leading to a return to a more normal health status. Without MePA in the human diet, physiological maintenance breaks down, leading to a reduction in general health status. Current evidence argues persuasively that MePA is an essential nutrient needed to maintain normal health and physiological function in humans.
is normally not produced within the body in amounts adequate to meet normal physiological needs,
– There is no endogenous synthesis of MePA in the body, with the only organisms believed to have genetic capability for endogenous synthesis being select marine microorganisms.[10] Since MePiA and MePA are related through oxidation-reduction reactions, generation of MePA within the body from MePiA is possible. However, there is no natural dietary source of MePiA available today.
and which causes, by its absence or underutilization, a clinically defined deficiency syndrome.
– Absence of dietary MePA contributes as a deficiency disease to the clinically defined syndrome of modern human aging. A clinical description of human aging states that "human aging affects all physiological processes".[11] Therefore, if dietary MePA deficiency is truly a player in the human aging syndrome, restoration of MePA to the diets of humans suffering with the aging syndrome has potential to positively impact "all physiological processes". So far, positive impacts of dietary supplementation with MePA on the nervous system, integumentary system, circulatory system, immune system, reproductive system, and respiratory system have been reported, with, importantly, some instances of temporary return of specific aspects of the aging syndrome when MePA supplementation was reduced or temporarily stopped.[12] With a gradual abatement of the diverse physiological decline characteristic of the human aging syndrome when dietary MePA is present and a gradual return of diverse physiological decline characteristic of the aging syndrome without (sufficient) dietary intake of MePA, the evidence supports the conclusion that there is a cause and effect relationship between the absence of dietary MePA and the advance of the clinically defined aging syndrome.
Additional evidence indicating that MePA is a vitamin is provided by the observation that the physiologically active intake range is very low, and, simultaneously, its toxicity is also very low. This is a distinctive characteristic of many of the vitamins, with 6 out of the 13 traditional vitamins having μg per day recommended intakes, but limited toxicity.[13],[14] Vitamin B12 is a particularly striking example. Vitamin B12 has the lowest recommended daily intake of any of the vitamins (2.4 μg per day for an adult male) but has no apparent oral toxicity in humans[15] and with acute oral toxicity of {>}5000 mg/kg body weight (BW) in mice[16]. To obtain an objective measure of toxicity in relation to intake level, if an acute oral toxicity of 5 g/kg BW is assumed, then 5g/kg BW multiplied by a 73 kg BW divided by 2.4 μg per day yields a safety margin of 152 million for intake of vitamin B12 for an adult male. The physiologically responsive range for MePA is on par with that of vitamin B12, with intakes as low as 1 μg per day eliciting marked physiological response.[17] When an LD50 of 1888 mg/kg BW[18] is used in conjunction with a recommended daily intake of 20 μg per day, then the safety margin for intake of MePA is 6.9 million. These very low recommended intake levels coupled with very large safety margins for vitamin B12 and MePA are not characteristic of most drugs. Most drugs require intakes in the mg per day range in order to elicit the desired physiological response, however, there are a limited number of drugs that are capable of a physiological response at very low doses. For example, the drug carfentanil can elicit a response in humans starting around 1 μg.[19] However, a general characteristic of these extremely potent drugs is that the range of safe intake levels around the physiologically responsive range is very small. For example, while human toxicity data is limited, carfentanil is potentially lethal at a dose of 20 μg.[20] Therefore, the safety margin for intake of carfentanil is about 20 relative to the minimum amount needed to see a physiological response. Therefore, while MePA shares the μg range physiologically responsive intake with both vitamin B12 and the drug carfentanil, it does not have the small safety margin property of carfentanil, but rather has the large safety margin property of vitamin B12. This provides additional evidence that MePA is a vitamin.
There is an extensive history of thousands of years of (unwitting) use of the MePiA–MePA duo as dietary ingredients from ancient historical life span data.[21] Of particular interest from these data in the present context is the observation that, for an interval of approximately 70 years, the natural intake of MePiA–MePA was abnormally high (probably on the order of 100 μg/day or more) with no apparent negative health consequences and significant positive health consequences resulting in increased life spans.[22]
There is a modest history of use of MePA by itself (without MePiA present) with modern volunteer testers. MePA was first tested by two individuals, both seniors in their early sixties, one male and one female.[23] The male began taking 1 μg MePA per day on November 26, 2015. The female began taking 1 μg MePA per day on November 7, 2016. On June 9, 2017 both individuals increased the intake from 1 to 2 μg per day per person. Significant physical and mental health benefits ascribed to MePA were reported by both of these individuals. Both individuals continued to use MePA by itself, slowly titrating upward, until mid-July 2019, at which point they began to supplement with 50:50 v/v aqueous MePA:MePiA. No adverse effects have been observed or reported by either individual. MePA, by itself, was tested by several dozen volunteers beginning mid-July 2017 and ending mid-July 2019. Many physical and mental health benefits were reported, and no adverse effects were reported. Testing of MePA by itself was replaced by testing of 50:50 v/v aqueous MePA:MePiA beginning late in July 2019 and continuing to the present time. The group of voluntary testers has increased to several hundred. The pattern of reports of health benefits with no adverse effects continues.
There is no known risk to health, from either theory or experience, due to inclusion of MePA in the diet in tens of μg amounts per day. That dietary supplementation with μg amounts of MePA per day may "reasonably be expected to be safe," as required by Sec. 190.6, is assured by the smallness of the daily intake, in combination with the water solubility of MePA.
The acute oral toxicity of MePA was formally evaluated via standard LD50 tests in laboratory rats.[24] The results showed an LD50 of 1888 mg/kg body weight (BW) with a 95% confidence interval of 1462 to 2438 mg/kg BW. This places the acute oral toxicity of MePA 3.8 times lower than the 501 mg/kg BW minimum required for a compound to be considered to be in the low acute oral toxicity category.[25] For general reference on the toxicity scale, comparing to other oral LD50 values in rats, the LD50 of MePA is 1.6 times more toxic than table salt (NaCl)[26], and 9.8 times less toxic than caffeine[27] (Figure 2).
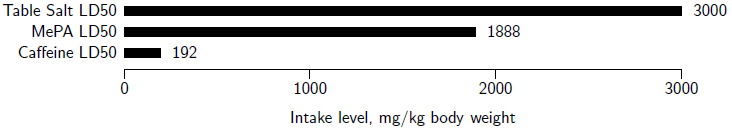 |
In addition to being of low acute oral toxicity, MePA is not mutagenic.[28]
MePA does not display chronic toxicity. MePA will be highly dissociated at physiological pH based on its pKa values (pKa1 = 2.12 and pKa2 = 7.29), and will therefore not bioaccumulate over time. Experiments with mice (designed to look for increased longevity rather than toxicity) revealed no evidence of chronic oral toxicity. For example, 7 months of supplementation of the diets of 36 Hsd:ICR (CD-1®) female mice at 100 mg MePA/liter drinking water beginning at 5.7 months of age yielded neither morbidity nor mortality.[29] Assuming a daily water consumption of 2.5 liters, this is equivalent to an adult human intake of 250,000 μg MePA per day for 1/5th of the usual human life span.
The risk to public health of inclusion of MePA in the diet in adequate daily amounts relative to exclusion of MePA from the diet appears to be infinitesimal. Conversely, the risk to public health of exclusion of MePA from the diet relative to inclusion of MePA in the diet in adequate daily amounts appears to be large.
Logic and ethics combined insist that the focus of legitimate safety concerns, with this substance, lies not in toxicology due to its use but rather in metabolic disease due to its lack of use. As with any vitamin, an inadequate daily intake of MePA in the diet induces morbidity in diverse physiological systems. At present, MePA is seriously deficient in the global population, including the entire U.S. population. Correction of this deficiency has potential to rid the population of many age-related ailments, some of which are serious, widespread health issues. Currently available evidence makes reasonable the conjecture that deficiency of this vitamin is responsible, for example, for prevalence of some autoimmune diseases, including some types of CIDP and rheumatoid arthritis. It appears that correcting this deficiency disease should be a major health objective of public health officials everywhere. Because this substance is known, empirically, to be safe when taken in tens of μg amounts, and because currently available evidence both experimental and theoretical says overwhelmingly that it is a long-lost vitamin responsible for some of the symptoms of the aging syndrome as it is universally experienced today, the only action which promotes safety with respect to MePA is to afford the general public prompt, unrestricted access to it.
Ingredients for this dietary supplement are only three in number: (1) water, (2) MePA, and (3) MePiA. In addition, the supplement contains < 0.02% ethyl alcohol as a byproduct of MePiA synthesis.
Commercially available distilled water is used for ingredient 1. Because the dietary supplement is consumed by the end user at ≤320μL per day, use of the commercial distilled water product without further in-plant characterization and monitoring of water quality is sufficient to yield a final dietary supplement which may "reasonably be expected to be safe."[30]
Commercially available synthetic MePA is used for ingredient 2. The commercial supplier is MilliporeSigma (formerly Sigma-Aldrich). MilliporeSigma research grade MePA (product number 289868) has a certified assay of 98%. The maximum exposure to impurities in the dietary supplement is thus sub μg per day per person. This low level makes further effort to ensure against toxicity unreasonable. Ambient background levels of exposure to uncharacterized compounds—from the air we breathe, the water we drink, the food we eat, the surfaces we touch, etc—is much greater than this. For example, the mass concentration of aerosols in continental tropospheric air exceeds 10 μg/m3. Given a conservative air volume breathed of 10,000 liters per person per day yields an uncharacterized aerosol exposure of 100 μg per person per day. Thus, impurities present in research grade MePA will not add significantly to an individual's unavoidable ambient background exposure load when the dietary supplement is ingested in the amounts specified for the supplement. Use of MilliporeSigma's commercially available product thus yields a final dietary supplement which may "reasonably be expected to be safe."[31].
The low concentration of MePA in the dietary supplement (maximum of 1.25 mg per bottle) coupled with the very low toxicity of MePA means that to reach an intake of even 1% of the acute oral LD50 of MePA discussed previously (1888 mg/kg BW), an adult male (73 kg BW) would need to consume in excess of 1,000 bottles (roughly 20 liters) of the dietary supplement. The intake of 20 liters of water required to consume 1,000 bottles of the dietary supplement places physical limits on the quantity of the dietary supplement that can be consumed at one time. This means that even abusive intake (e.g., disregarding the recommended daily intakes for the dietary supplement and megadosing), can "reasonably be expected to be safe."[32]
Safety of the third ingredient, MePiA, is discussed in a separate NDIN accompanying this one.
In-plant Quality Control will check the concentration of MePA in mixed batches of the dietary supplement via ion chromatography to ensure a final concentration within 10% of the targeted 62.5 mg MePA per liter of supplement prior to final bottling of the dietary supplement. ◇
The Biblical Chronologist is written and edited by Gerald E. Aardsma, a Ph.D. scientist (nuclear physics) with special background in radioisotopic dating methods such as radiocarbon. The Biblical Chronologist has a fourfold purpose: to encourage, enrich, and strengthen the faith of conservative Christians through instruction in biblical chronology and its many implications, to foster informed, up-to-date, scholarly research in this vital field, to communicate current developments and discoveries stemming from biblical chronology in an easily understood manner, and to advance the growth of knowledge via a proper integration of ancient biblical and modern scientific data and ideas. The Biblical Chronologist (ISSN 1081-762X) is published by: Aardsma Research & Publishing Copyright © 2020 by Aardsma Research & Publishing.
|
^ Sec. 190.6 (a) and (b)(4).
^ www.biblicalchronologist.org/products/vitamin_MePA_ NDIN_response.php.
^ biblicalchronologist.org/products/FDA_Journal.php. See especially the Monday, June 4, 2018 entry.
^ Piro A, Tagarelli G, Lagonia P, Tagarelli A, Quattrone A: Casimir Funk: His Discovery of the Vitamins and Their Deficiency Disorders. Ann Nutr Metab 2010;57:85-88. doi: 10.1159/000319165
^ Walter H. Eddy, Vitaminology: The Chemistry and Function of the Vitamins (Baltimore: The Williams & Wilkins Company, 1949), Foreword.
^ Physicians' Vitamin Reference Book, third edition (New York: E.R. Squibb & Sons, January 1940), 46–47.
^ fda.gov/media/99538/download, page 99.
^ Gerald E. Aardsma, Aging: Cause and Cure (Loda, IL: Aardsma Research and Publishing, 2017), pages 79-89. www.BiblicalChronologist.org.
^ https://www.biblicalchronologist.org/products/vitamin_M ePA_testimonials.php.
^ Metcalf, William W., Griffin, Benjamin M., Cicchillo, Robert M., Gao, Jiangtao, Janga, Sarath Chandra, Cooke, Heather A., Circello, Benjamin T., Evans, Bradley S., Martens-Habbena, Willm, Stahl, David A., van der Donk, Wilfred A., Synthesis of methylphosphonic acid by marine microbes: A source for methane in the aerobic ocean. Science, Vol 337(6098), pages 1104-1107, 2012.
^ Boss, G. R., Seegmiller, J. E., Age-related physiological changes and their clinical significance. The Western Journal of Medicine, Vol 135(6), pages 434-440, 1981.
^ https://www.biblicalchronologist.org/products/vitamin_M ePA_testimonials.php.
^ Gropper, S. S., Smith, J. L., Groff, J. L., The Water-Soluble Vitamins. In: Advanced Nutrition and Human Metabolism. 5th ed. Belmont, CA: Wadsworth, Cengage Learning; 2009. pages 309-372.
^ Gropper, S.S, Smith J. L., Groff, J. L., The Fat-Soluble Vitamins. In: Advanced Nutrition and Human Metabolism. 5th ed. Belmont, CA: Wadsworth, Cengage Learning; 2009. pages 373-427.
^ Gropper, S. S., Smith, J. L., Groff, J. L., The Water-Soluble Vitamins. In: Advanced Nutrition and Human Metabolism. 5th ed. Belmont, CA: Wadsworth, Cengage Learning; 2009. pages 309-372.
^ https://pubchem.ncbi.nlm.nih.gov/compound/5311498# section=Toxicity-Summary, accessed June 9 2020.
^ Gerald E. Aardsma, Aging: Cause and Cure (Loda, IL: Aardsma Research and Publishing, 2017), pages 113-118. www.BiblicalChronologist.org.
^ Watson, Rebecca E., Hafez, Ahmed M., Kremsky, Jonathan N., Bizzigotti, George O. Toxicity of binary chemical munition destruction products: Methylphosphonic acid, methylphosphinic acid, 2-diisopropylaminoethanol, DF neutralent, and QL neutralent. International Journal of Toxicology, Vol 26, pages 503-512, 2007.
^ https://pubchem.ncbi.nlm.nih.gov/compound/62156, accessed June 6 2020.
^ https://pubchem.ncbi.nlm.nih.gov/compound/62156#sec tion=Human-Toxicity-Excerpts, accessed June 6 2020.
^ Gerald E. Aardsma, Aging: Cause and Cure (Loda, IL: Aardsma Research and Publishing, 2017), Chapter 2, pages 27–34. www.BiblicalChronologist.org.
^ See, for example, Gerald E. Aardsma, Aging: Cause and Cure (Loda, IL: Aardsma Research and Publishing, 2017), page 104. www.BiblicalChronologist.org.
^ Gerald E. Aardsma, Aging: Cause and Cure (Loda, IL: Aardsma Research and Publishing, 2017), Chapter 15, pages 113–118. www.BiblicalChronologist.org.
^ Watson, Rebecca E., Hafez, Ahmed M., Kremsky, Jonathan N., Bizzigotti, George O. Toxicity of binary chemical munition destruction products: Methylphosphonic acid, methylphosphinic acid, 2-diisopropylaminoethanol, DF neutralent, and QL neutralent. International Journal of Toxicology, Vol 26, pages 503-512, 2007.
^ Munro, N. B., Talmage, S. S., Griffin, G. D., Waters, L. C., Watson, A. P., King, J. F., Hauschild, V. The sources, fate, and toxicity of chemical warfare agent degradation products. Environmental Health Perspectives, Vol 107(12), pages 933-974, 1999.
^ https://pubchem.ncbi.nlm.nih.gov/compound/5234#sec tion=Non-Human-Toxicity-Values, accessed June 1 2020.
^ https://pubchem.ncbi.nlm.nih.gov/compound/2519#sec tion=Acute-Effects, accessed June 1 2020.
^ Watson, Rebecca E., Hafez, Ahmed M., Kremsky, Jonathan N., Bizzigotti, George O. Toxicity of binary chemical munition destruction products: Methylphosphonic acid, methylphosphinic acid, 2-diisopropylaminoethanol, DF neutralent, and QL neutralent. /textit{International Journal of Toxicology} Vol 26, pg 503-512, 2007.
^ Gerald E. Aardsma, Aging: Cause and Cure (Loda, IL: Aardsma Research and Publishing, 2017), page 112. www.BiblicalChronologist.org.
^ Sec. 190.6 (a) and (b)(4).
^ Sec. 190.6 (a) and (b)(4).
^ Sec. 190.6 (a) and (b)(4).