
 | |
| Volume 12, Number 1 | April 26, 2022 |
The present RDI (Recommended Daily Intake) of the anti-aging vitamins, MePiA (methylphosphinic acid) and MePA (methylphosphonic acid), is 20 μg (micrograms) of each vitamin for adult males and 15 μg of each vitamin for adult females.[1] This recommendation was based on a "confidently enough" policy designed to ensure that Dr. Aardsma's Anti-Aging Vitamins dietary supplement was providing consumers with physiologically sufficient amounts of these two vitamins.
MePA excreted in the urine of an adult female volunteer supplementing her diet with Dr. Aardsma's Anti-Aging Vitamins at the present RDI for many months has now been measured. The amount of MePA excreted in a total 24-hour urine sample was found to be 13±1.4 μg.[2]
The fact that MePA is being excreted implies that the present RDI is sufficient to more than meet the physiological demands for vitamin MePA in this adult female. If the body needed more MePA than was being supplied by dietary supplementation, then no MePA would have been excreted.
Known dietary sufficiency of MePA does not guarantee dietary sufficiency of MePiA. To guarantee dietary sufficiency of MePiA requires independent measurement of excreted MePiA, which has not yet been accomplished. However, the extraordinarily long biological half-life of MePiA (135 years)[3] suggests that the daily intake of this vitamin needed to meet physiological demands is likely to be extremely small, and this implies that the daily demand for MePA is likely to be larger than the daily demand of MePiA. Since Dr. Aardsma's Anti-Aging Vitamins dietary supplement supplies both vitamins in equal amounts, the present confirmation of its sufficiency with respect to MePA lends substantial weight to the expectation of its sufficiency with respect to MePiA as well.
The present article thus affirms success of the "confidently enough" policy and documents the experimental measurement method and results yielding this affirmation.
Electrospray ionization (ESI) coupled with tandem mass selective quadrupoles (i.e., tandem mass spectrometry or MS-MS) was used for measurement of MePA.[4] ESI in its negative ionization mode yields methylphosphonate (MePA-) ions. These negative ions have a mass of 95.0071 AMU. The first quadrupole was tuned to transmit this mass. Upon emergence from the first quadrupole, electrons were stripped from the negative ions via collision with argon gas atoms, causing the ions to break up into fragments. The dominant positive ion fragment was found to be of mass 79 AMU, corresponding to PO3+ (78.97 AMU). The second quadrupole was tuned to transmit this positive ion to the ion detector. Highly sensitive and selective detection of MePA was achieved in this way.
Frequent spikes were observed to be present in the electronic background noise spectrum of the mass spectrometer. These were discriminated against during mass spectrum accumulation using the software spike removal utility provided with the mass spectrometer.
ESI was found not to produce reliably reproducible negative ion currents. It was therefore necessary to spike the sample prior to measurement with a known amount of a standard to accurately quantitate MePA. For this purpose, isotopically labeled MePA was used. Commercially available MePA having CD3 in place of the usual CH3 methyl group was used.[5] This standard (call it MePA*) has a mass of 98.0257 AMU. MePA in the sample is measured relative to the known MePA* in the sample. MePA shows up in the ESI-MS-MS detector as a peak in the parent-ion mass spectrum centered near 95 AMU and MePA* shows up as a peak centered near 98 AMU.
When a sample of urine highly diluted with deionized water (DIW) was analyzed via ESI-MS-MS, the resulting parent-ion mass spectrum in the region of interest to MePA was overwhelmingly dominated by an intense peak at roughly 97 AMU. This mass corresponds to the singly charged dihydrogenphosphate ion, H2PO4-. Among the fragments resulting from collision of this ion with argon gas following the first quadrupole are PO3+ ions, just as with MePA-, which is why it appears in the parent-ion mass spectrum between MePA at mass 95 and MePA* at mass 98.
Phosphate is ubiquitous, having a natural presence in biological samples at concentrations many orders of magnitude greater than the micrograms of MePA per liter of interest to vitamin MePA measurements. To measure MePA in urine accurately, it was necessary first to reduce the concentration of phosphate in the measurement sample by a large amount in some way.
For this purpose, high pressure ion chromatography (HPIC) was used. The instrument used was a Dionex DX-100 equipped with a carbonate removal device and a 4 mm × 250 mm AS25 column. The eluent was 2 ml of 50% NaOH per liter of DIW, operated at a flow rate of 1 ml/minute. The suppressor and carbonate removal device were continuously purged with DIW at 2 ml/minute. MePA (including MePA*) was fraction collected, reducing phosphate in the sample to manageable proportions.
Injection of raw urine onto the AS25 column was found to result in rapid deterioration of the column. It was therefore necessary to prep the urine sample to remove compounds incompatible with the AS25 column prior to HPIC processing.
Solid phase extraction (SPE) was used for this purpose. Raw urine was loaded onto a formate-form, Poly-Prep® AG® 1-X8 column[6] and then eluted with 1 M HCl to yield a HPIC-ready sample containing just the reasonably-rapidly-eluting anions from the urine in an aqueous solution of formic acid (relatively minor, from the formate-form resin) and HCl (major, from the 1 M HCl SPE eluent).
Figure 1 shows a typical HPIC chromatogram.
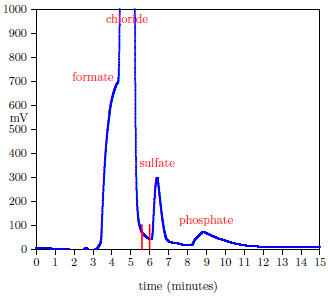 |
The stainless steel ESI probe tip was found to be a source of sample-to-sample memory contamination. This contamination was eliminated by 5 minutes of ultrasonication of the probe tip in 80 C distilled water prior to each measurement.
The total volume of the 24-hour urine collection was recorded. A 2 ml sample was drawn from the 24-hr collection and spiked to yield 100 μg MePA* per liter. This spiked sample was loaded onto the SPE resin bed, washed with DIW, then eluted first with 3.5 ml of 1 M HCl and then with an additional 2 ml of 1 M HCl simultaneous with collection of a 2 ml fraction. This fraction was loaded into the HPIC autosampler, and 22 repeat injections of 25 μL each injection were made onto the IC column at 1 hour intervals. A 0.4 ml fraction was collected following the conductivity detector beginning typically at 5.6 minutes after the start of each injection. The resulting total collection of roughly 9 ml was then concentrated to approximately 1 ml by blowdown with air in a 50 C oven. To the concentrated sample was added 1 ml of MeOH, yielding the ESI-ready sample. ESI was run at 2.4 kV (limited by onset of corona discharge at the probe tip) at a flow rate of 10 μL per minute. Desolvation gas was 400 L/hr of dry air at 350 C. A parent-ion mass spectrum was accumulated over the range of masses from 90.5 to 105.5. The single-scan time for this range was 1 s with a delay of 0.05 s between scans. The entire approximately 2 ml sample was utilized, resulting in a run time for accumulation of the mass spectrum of roughly 3 hours.
The accumulated mass spectrum, stored as a binary file by the mass spectrometer's software (MassLynx), was processed using a custom Fortran program. This used a simple least squares procedure to fit a sum of 14 flat-topped Gaussian peaks plus a constant background to the raw mass spectral data beginning with mass 92. Flat-topped Gaussian peaks of the form
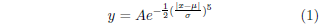
were found to give a reasonable match to the observed shape of the mass spectral peaks. The values of μ were fixed parameters specific to each mass peak, while σ was a single free parameter equal for all mass peaks. The program returned the mass 95 (MePA) to mass 98 (MePA*) ratio of interest by simply dividing the amplitudes of the respective Gaussian peaks.
A calibration curve for MePA in urine measured using this protocol was constructed using urine from a male volunteer not taking Dr. Aardsma's Anti-Aging Vitamins. Calibration samples were prepared as detailed above. These were spiked with known concentrations of MePA at the same time they were spiked with MePA*. Table 1 shows the result of the calibration sample runs.
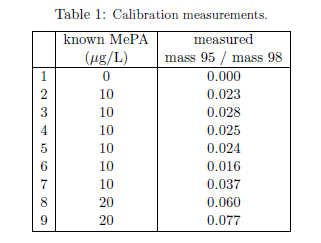 |
The first line of the table shows the result for a urine sample to which no MePA was added. This resulted in no mass 95 peak. The only peaks in the recorded mass spectrum for this sample were mass 97, from residual phosphate, and mass 98, from the usual spike of MePA*. This demonstrated the general freedom from background peaks afforded by this protocol (Figure 2).
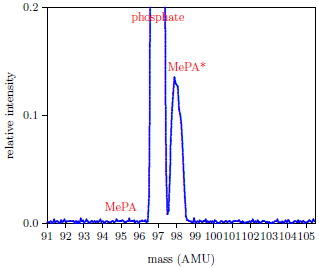 |
The second line of Table 1 shows the result for a urine sample spiked with 10 μg of MePA per liter of urine. The standard spike in this sample was 100 μg MePA* per liter of urine, as usual. The ratio of the mass 95 peak area to the mass 98 peak area is not simply (10/100=) 0.1 because the fragmentation patterns of MePA and MePA* in argon gas are not the same. Because MePA and MePA* have different masses, they enter the argon gas after the first quadrupole with different velocities, and this alters subsequent collision dynamics. In addition, the more massive CD3 is much more easily removed from MePA* than is the less massive CH3 of MePA.
A linear calibration curve passing through the origin was least squares fit to the "mass 95 / mass 98" versus "known MePA" data of Table 1. This gave a slope of 0.00305 ± 0.00026.
Three samples of the unknown, 24-hour urine sample were measured. These gave 0.031, 0.024, and 0.026 as the ratio of the mass 95 to mass 98 peak areas. The average of these three measurements is 0.027±0.0036. This average yields, by means of the calibration curve calculated above, 13±1.4 as the total MePA excreted in the 24-hour collection interval.
It is now clear that MePA is being excreted at the present RDI. This is the important result, guaranteeing that consumers of Dr. Aardsma's Anti-Aging Vitamins dietary supplement are getting sufficient MePA to meet physiological demands.
A second important implication of the present result is confirmation of the theory leading to the present RDI. The present RDI was expected to be on the high side of actual physiological requirement, and this is what has now been found to be the case.
A further important consequence is that the demonstrated ability to accurately measure excreted MePA places the RDI for this vitamin on an empirical footing for the first time. This in turn enables further refinement of this important quantity into the future.
Improvements of the measurement method are expected to be ongoing. This first measurement makes it seem probable that the RDI for MePA will ultimately be adjusted downward. Improvements to the measurement method will be essential should the daily physiological demand for MePA turn out to be submicrogram. ◇
The Biblical Chronologist is written and edited by Gerald E. Aardsma, a Ph.D. scientist (nuclear physics) with special background in radioisotopic dating methods such as radiocarbon. The Biblical Chronologist has a fourfold purpose: to encourage, enrich, and strengthen the faith of conservative Christians through instruction in biblical chronology and its many implications, to foster informed, up-to-date, scholarly research in this vital field, to communicate current developments and discoveries stemming from biblical chronology in an easily understood manner, and to advance the growth of knowledge via a proper integration of ancient biblical and modern scientific data and ideas. The Biblical Chronologist (ISSN 1081-762X) is published by: Aardsma Research & Publishing Copyright © 2022 by Aardsma Research & Publishing.
|
^ Matthew P. Aardsma, "Intake Recommendations for Dr. Aardsma's Anti-Aging Vitamins," The Biblical Chronologist 10.10 (June 10, 2020): 1–8. www.BiblicalChronologist.org.
^ This is the mean plus or minus one standard deviation.
^ Gerald E. Aardsma and Matthew P. Aardsma, Aging: Cause and Cure, 2nd ed. (Loda, IL: Aardsma Research and Publishing, 2021), 110. www.BiblicalChronologist.org.
^ The specific instrument used was a Waters Quattro micro API mass spectrometer.
^ Purchased from Cambridge Isotope Laboratories, Inc.
^ Purchased from Bio-Rad.