
 | |
| Volume 13, Number 10 | December 19, 2023 |
The biblical book of Exodus records that a pillar of cloud during the daytime and a pillar of fire at night (Figure 1)[1] accompanied the Israelites as they journeyed to the Promised Land following their departure from Egypt.
Then they set out from Succoth [the first encampment] and camped in Etham on the edge of the wilderness. And the Lord was going before them in a pillar of cloud by day to lead them on their way, and in a pillar of fire by night to give them light, that they might travel by day and by night. He did not take away the pillar of cloud by day, nor the pillar of fire by night, from before the people. (Exodus 13:20–22)A scientific explanation of the pillar of cloud has been provided in an earlier article.[2] There it was shown how application of modern scientific principles and methods to the biblical narrative taken simply at face value leads to a purely naturalistic explanation of the pillar of cloud. Modern science reveals that several million people, camped in the naturally dry savanna environment which prevailed back at the time of the Exodus (2450 B.C.), would necessarily have caused such a pillar of cloud. The present article goes on to show a modern scientific explanation of the pillar of fire. An interesting consequence of this explanation is ability to know, at present, nearly four and a half thousand years after the Exodus took place, the color of the pillar of fire.
 |
The pillar of fire does not seem to have been a pillar of ordinary fire. There is no record of any smoke accompanying it, for example. This contrasts with the description of the fire which occurred on Mount Sinai while the Israelites were camped there.
Now Mount Sinai was all in smoke because the Lord descended upon it in fire; and its smoke ascended like the smoke of a furnace, and the whole mountain quaked violently. (Exodus 19:18)Clearly, the text has no trouble recording the presence of smoke accompanying a fire. But in regard to the pillar of fire, there is never any mention of smoke.
This same Mount Sinai event also makes clear that the ancient biblical text has no difficulty describing and recording lightning.
So it came about on the third day, when it was morning, that there was thunder and lightning flashes and a thick cloud upon the mountain… (Exodus 19:16a)The "thick cloud" mentioned here is not the pillar of cloud. The pillar of cloud was caused by, and originated from, the people, camped on the plain below the mountain. The "thick cloud" mentioned here was caused by and originated from the theophanic impact event in which, the narrative records, God descended upon the mountain.[3]
Evidently, the pillar of fire was due neither to ordinary chemical burning of some fuel nor to electrical storm activity within the cloud.
But it is clear that the pillar of fire must have resembled fire. Most fundamentally, like fire, it produced light. Evidently, it produced sufficient light to see by while walking at night.
And the Lord was going before them in a pillar of cloud by day to lead them on their way, and in a pillar of fire by night to give them light, that they might travel by day and by night. (Exodus 13:21)
Clearly, there was light coming from the cloud. The cloud glowed at night. But it did not glow with a white light, like one might see with flashes of heat lightning or with lightning inside a cloud. The text describes it as a pillar of "fire," not a pillar of light. Evidently, the pillar of fire glowed as if its light came from a fire. This suggests that the cloud glowed with a color from the red end of the spectrum.
Is there any way, known to science, by which a fire-reddish light not due to normal chemical burning or electrical discharge might be obtained in nature?
Yes. Chemiluminescence.
Chemiluminescence has been made familiar today by the commercial availability of glow sticks (also called light sticks, light wands, or chem sticks). These can be purchased in a variety of colors. A glow stick contains two liquids, one in an inner, sealed, glass vial and another in the outer, flexible, plastic tube making up the "stick." When the stick is bent, breaking the glass vial, the two fluids mix together. When the fluids mix, they react chemically to produce light. This light, from chemical reaction, is called chemiluminescence.
I suggest that the pillar of fire glowed by chemiluminescence. The chemiluminescent reaction in this case took place within the water droplets making up the cloud.
Different chemiluminescent chemicals produce different colors. The actual chemical substance producing light in the water droplets of the pillar of fire, I suggest, was singlet oxygen.
Singlet oxygen has a confusing name. There is nothing "singlet" about it to ordinary folk. It is just a regular oxygen molecule, O2, with some extra internal energy in its electron orbitals. I will use an asterisk to show the extra energy, writing singlet oxygen like this: O2*.
Chemiluminescence results from a chemical reaction producing singlet oxygen when singlet oxygen gives off its extra electron orbital energy as light.
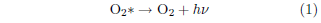
In this equation, hν represents a quantum of light, called a "photon."
The color of a photon is determined by its wavelength. In Equation 1, the photon which is given off has a wavelength of 1270 nanometers (nm).[4] This is in the infrared portion of the electromagnetic radiation spectrum, which is not visible to the human eye. Clearly, this particular chemiluminescence is not of interest in the present context.
There is, however, another chemiluminescence involving singlet oxygen which is of interest. It results from two singlet oxygen molecules joined together, forming a dimer of singlet oxygen.

This reaction can produce two different photons. The most probable case is emission of a 703-nm photon. This is a red photon. You can see the color corresponding to this wavelength by entering "703" (without the quotes) in the "Wavelength" field at 405nm.com/wavelength-to-color/. A less probable case is emission of a 633-nm photon. This is an orange photon. These would have been the colors of the photons coming from the pillar of fire according to the singlet oxygen theory of the pillar of fire presented here.
The Singlet Oxygen Theory of the Pillar of Fire: The pillar of fire was the pillar of cloud at night illuminated by chemiluminescence from dimers of singlet oxygen within its water droplets.
When photons of two different colors and intensities enter our eyes simultaneously, they are summed by our eyes, giving a perceived color different from the colors of the individual photons. Thus, to learn the color of the pillar of fire as closely as possible, it is easiest simply to view the chemiluminescence which is produced when the Equation 2 reaction is carried out in the laboratory. There are several videos on the Internet showing chemiluminescence from the Equation 2 reaction of singlet oxygen. I recommend "The preparation of singlet oxygen."[5] The chemical reaction being used to produce O2* in this video is not the same as would have been operating within the pillar of cloud, but the color of the light coming from the reaction would have been the same in both instances since chemiluminescence of singlet oxygen always produces the same color photons. From 2:38 to 3:38 minutes (best viewed full screen), this video inadvertently illustrates how much the chemiluminescence of O2* looks like fire. What could easily be mistaken for flames of fire blowing about in the wind during this segment of the video is actually chemiluminescence from dimers of singlet oxygen produced as chlorine gas bubbles up through a solution of basic hydrogen peroxide from an underlying porous glass frit. There is no fire. The whole apparatus, including the fire-red chemiluminescence, is at room temperature.
To this point, support for the singlet oxygen theory of the pillar of fire has been garnered from two facts. First, the pillar of fire does not seem to have produced any smoke. As noted above, this argues against conventional fire while being supportive of chemiluminescence as the source of the light coming from the pillar of fire. Second, chemiluminescence of the dimer of singlet oxygen yields photons producing a color which looks suitably like a glow due to fire, as the video discussed above shows.
Further support comes first of all from the presence within the pillar of fire of a suitable solvent—water (as cloud droplets)—in which the chemical reactions necessary to produce chemiluminescence might be operative.
This is augmented by the observation that the appearance of fire pertained only to the pillar of fire—the nighttime air of the camp did not appear to be on fire. This implies that it was the liquid cloud droplets, not the air, which sourced the glow coming from the pillar of fire, in harmony with the idea of aqueous chemiluminescent reactions.
Additional support comes from the presence of the chemical reactants needed to generate chemiluminescence from dimers of singlet oxygen within the pillar-of-fire cloud droplets.
Reactants common to glow stick chemiluminescence include a base which acts to catalyze the reaction, and the reactive oxygen species hydrogen peroxide (H2O2).[6] These same two ingredients were present in the reaction carried out in "The preparation of singlet oxygen" video referenced above to show the color of chemiluminescence from the dimer of singlet oxygen. The base used in the video was supplied by a 20% sodium hydroxide solution, and it was mixed with a 15% H2O2 solution.
The need for a basic, rather than an acidic, solution for production of chemiluminescence is quite convincing of the idea that chemiluminescence was the source of illumination in the pillar of fire. Normally, cloud droplets are acidic because of the presence in air of carbon dioxide which reacts in water to produce carbonic acid. But the air in the Israelite camps was unusually rich in ammonia gas due to the vast herds of sheep and other livestock which they kept.[7] Ammonia is readily absorbed by water droplets. It acts as a base when it dissolves in water, yielding ammonium ions and hydroxide ions. The pillar of fire was comprised of the ammonia-rich air coming from the Israelite camps. Thus basic, rather than the normally acidic, cloud droplets were uniquely possible within the pillar of fire.
Hydrogen peroxide is a common atmospheric trace gas. It would have been naturally present in the pillar of fire droplets.
Hydrogen peroxide is also an important compound for atmospheric chemistry, particularly in the liquid phase. It has few direct emission sources, but is chemically produced in the gas phase by HO2· disproportionation and in the liquid phase by a variety of homogeneous [reactions involving only dissolved substances] and heterogeneous [reactions involving solid particulates together with dissolved substances] processes.[8]Hydrogen peroxide is very water soluble, so it can be expected to be absorbed from air into cloud droplets whenever cloud droplets are present. Thus the pillar of fire cloud droplets have potential to supply a basic hydrogen peroxide solution suitable to singlet oxygen formation.
Like hydrogen peroxide, singlet oxygen is expected to be produced in cloud droplets by a variety of homogeneous and heterogeneous processes. For example:
Interestingly, [steady state singlet oxygen concentration] in aqueous road dust extracts was lower than in the corresponding particle-containing samples, which implies that the particle surface itself also participated in 1O2 [i.e., singlet oxygen] production.[9]
By way of homogeneous reactions producing singlet oxygen within the pillar of fire cloud droplets, it is possible, for example, that nitric oxide (NO) may have been a major player. The reaction of NO with H2O2 was observed, several decades ago, to produce singlet oxygen.[10]
This possibility is encouraged by the fact that NO, an atmospheric trace gas naturally present throughout the atmosphere, reaches its greatest natural abundance, roughly 10 parts per billion, over grasslands.[11] The route of the Exodus, which today traverses bare-earth desert, traversed savanna desert back at the time of the Exodus, 2450 B.C., due to less aridity back at that time than prevails today.[12]
At night, NO reacts with ozone (O3) to yield nitrogen dioxide (NO2). NO2 reacts in turn with O3 to yield nitrate radical (NO3). These same reactions can happen during the daytime, but sunlight rapidly breaks NO2 and NO3 back down to NO. Furthermore, NO3 reacts rapidly with abundant daytime NO to produce two NO2 molecules. Thus, while daytime air for the Israelites would have had significant NO with little NO3, the opposite would have been the case at night.
Now NO3 is about a thousand times more soluble in water than NO. This means that NO3 concentrations in water droplets at night may have been much larger than NO concentrations in water droplets during the daytime. Meanwhile, the reactions of these nitrogen species with ozone are reversible, which means that NO3 has potential to produce NO in water droplets at night, and this NO has potential to react with the basic H2O2 cloud droplet solution to produce singlet oxygen at night.
This scheme serves only as an example of potential homogeneous reactions yielding singlet oxygen. I have not investigated it quantitatively, so cannot say how important or otherwise it may have been in the case of the pillar of fire. It is interesting partly because it would have operated during the night but not during the day, providing a potential explanation for the lack of any report of chemiluminescence from the pillar of cloud during the day. But this may also possibly be explained by the simple fact that daylight may have made ongoing chemiluminescence from the pillar of cloud impossible to see just as it makes starlight impossible to see.
One should not think of any single cloud droplet as an intense source of chemiluminescence. In fact, the chemiluminescent intensity from any single droplet may have been too weak to see. The pillar of fire was a distributed light source, not a point light source.
The glow coming from the pillar of fire at any given moment would have resulted from the sum of chemiluminescent photons being emitted by all of the water droplets making up the pillar of fire during that moment. In a typical fog or low-lying cloud, one may expect on the order of 100 droplets per cubic centimeter.[13] This means that there would have been roughly one hundred million droplets emitting chemiluminescent light photons per cubic meter of cloud.
One should also not suppose that the pillar of fire chemiluminescence would have slowly dimmed and gone out the way a glow stick does. The pillar of fire chemiluminescence would have been continuous rather than episodic.
The cloud itself was being continuously renewed by an air circulation pattern centered on the Israelite camp.[14] Dry desert air was continuously flowing in from the edges of the camp while moistened camp air was continuously ascending from the center of the camp. A parcel of air flowing into the base of the pillar of cloud a few meters above the ground would begin to cool as it rose. This would cause it to begin to condense water droplets around particulates such as dust acting as condensation nuclei. These water droplets would absorb ammonia and hydrogen peroxide from the air parcel as it rose. Eventually, concentrations of reactants producing singlet oxygen in the water droplets in the air parcel would become large enough for chemiluminescence from the parcel to begin to be visible. Chemiluminescence would continue until one or more reactants had been used up. But by that time, the parcel of air would have ascended high above the camp. While the chemiluminescence of a single parcel of air would have been transitory, the chemiluminescence from the pillar of cloud would have been continuous because the cloud was continuously being renewed by new parcels of air entering it at its base.
The biblical pillar of fire, when assessed using modern principles and methods of science, appears to have been a natural phenomenon—a vertical convective cloud lit up from within by the fire-red chemiluminescence characteristic of dimers of singlet oxygen produced within the multitudinous water droplets comprising the cloud.
The presence of the pillar of cloud at the time of the Exodus seems to have been uniquely occasioned by 1) the millions of people plus their vast herds involved in the Exodus, 2) the savanna desert environment which existed in the Negev through which they traveled at that time, and 3) their camping lifestyle, with disposal of wastewater and urine on the surface of the ground.
The difficulties involved in bringing these necessary conditions for such a pillar of fire together in the modern world make it unlikely that this wonder of nature will ever be seen again. ◇
The Biblical Chronologist is written and edited by Gerald E. Aardsma, a Ph.D. scientist (nuclear physics) with special background in radioisotopic dating methods such as radiocarbon. The Biblical Chronologist has a fourfold purpose: to encourage, enrich, and strengthen the faith of conservative Christians through instruction in biblical chronology and its many implications, to foster informed, up-to-date, scholarly research in this vital field, to communicate current developments and discoveries stemming from biblical chronology in an easily understood manner, and to advance the growth of knowledge via a proper integration of ancient biblical and modern scientific data and principles. The Biblical Chronologist (ISSN 1081-762X) is published by: Aardsma Research & Publishing Copyright © 2023 by Aardsma Research & Publishing. Scripture quotations taken from the (NASB®) New American Standard Bible®, Copyright© 1960, 1971, 1977, 1995 by The Lockman Foundation. Used by permission. All rights reserved. www.Lockman.org |
^ www.freebibleimages.org/illustrations/moses-red-sea/
^ Gerald E. Aardsma, "Understanding the Pillar of Cloud," The Biblical Chronologist 13.8 (November 15, 2023): 1–19. www.BiblicalChronologist.org.
^ Gerald E. Aardsma, "The Crater at Mt. Yeroham – Part I," The Biblical Chronologist 9.1 (May 2008): 1–7. www.BiblicalChronologist.org.
^ Marina A. Tzani et al., "Direct and Indirect Chemiluminescence: Reactions, Mechanisms and Challenges," Molecules 26.24 (December 17, 2021): 7664.
^ www.youtube.com/watch?v=lUTE4ckMkYg
^ en.wikipedia.org/wiki/Glow_stick (accessed December 14, 2023).
^ Gerald E. Aardsma, Bread from Heaven: The Manna Mystery Solved (Loda, IL: Aardsma Research and Publishing, 2023). www.BiblicalChronologist.org.
^ T. E. Graedel, Donald T. Hawkins, and Larry D. Claxton, Atmospheric Chemical Compounds: Sources, Occurrence, and Bioassay, (Orlando, Florida: Academic Press, Inc., 1986), 60.
^ Chelsea D. Cote et al., "Photochemical Production of Singlet Oxygen by Urban Road Dust," Environmental Science & Technology Letters 5.2 (2018): 92-97.
^ Alberto A. Noronha-Dutra, Monica M. Epperlein, and Neville Woolf, "Reaction of nitric oxide with hydrogen peroxide to produce potentially cytotoxic singlet oxygen as a model for nitric oxide-mediated killing," FEBS Letters (1993): 321.
^ T. E. Graedel, Donald T. Hawkins, and Larry D. Claxton, Atmospheric Chemical Compounds: Sources, Occurrence, and Bioassay, (Orlando, Florida: Academic Press, Inc., 1986), 63.
^ Gerald E. Aardsma, Bread from Heaven: The Manna Mystery Solved (Loda, IL: Aardsma Research and Publishing, 2023), Appendix A. www.BiblicalChronologist.org.
^ John H. Seinfeld and Spyros N. Pandis, Atmospheric Chemistry and Physics (New York: John Wiley & Sons, Inc., 1998), 1178.
^ Gerald E. Aardsma, "Understanding the Pillar of Cloud," The Biblical Chronologist 13.8 (November 15, 2023): 1–8. www.BiblicalChronologist.org.