
 | |
| Volume 13, Number 9 | November 28, 2023 |
The biblical historical account of the Exodus reveals that the Israelite nation experienced zero population growth during the roughly forty years they lived in the desert.
A census of the nation is recorded shortly after they left Egypt, while they were camped at Mount Sinai:
So all the numbered men of the sons of Israel by their fathers' households, from twenty years old and upward, whoever was able to go out to war in Israel, even all the numbered men were 603,550. (Numbers 1:45–46)A census was taken again roughly forty years later, this time of the new generation, at the end of the nation's stay in the desert, while they were camped across the Jordan River opposite Jericho, just prior to the start of the Eviction/Conquest:
These are those who were numbered of the sons of Israel, 601,730. (Numbers 26:51)These two numbers reveal a slight overall decline in population while the Israelites lived in the desert.
This contrasts sharply with Israel's growth rate while they lived in Egypt. I have previously calculated this growth rate as follows:[1]
The Bible records that seventy persons accompanied Jacob at the start of the Israelites' stay in Egypt. (Genesis 46:27.) It also records that the duration of the Israelites' stay in Egypt was 430 years. (Exodus 12:40–41.) Two million people from seventy people after 430 years computes to an average population growth rate for the Israelites of about 2.4% per year. Wikipedia reports that the population growth rate for the world peaked at 2.2% per year in 1963.[2] We know that the rate of population growth for the Israelites in Egypt was large. Concern over it was, in fact, the whole basis of the Oppression. (Exodus 1:7–22.) So 2.4% per year for the Israelites in Egypt seems reasonable, …
Why did the population growth rate reduce from 2.4% prior to the Exodus to below zero after the Exodus while the Israelites lived in the desert?
Unfortunately, the biblical historical account of the Exodus nowhere explicitly addresses this question. It records several instances where thousands of Israelites died during the Exodus—3,000 at Sinai in connection with the golden calf incident (Exodus 32:28), 14,700 in connection with the rebellion of Korah (Numbers 16:49), and 24,000 in connection with the Baal of Peor idolatry (Numbers 25:1–9), for example. But the mathematics of population growth renders these losses relatively inconsequential. A growth rate of 2.4% per year for forty years would turn six hundred thousand fighting men into more than one and a half million fighting men. Thus, a deficit of some nine hundred thousand fighting men needs to be explained. Loss of a few tens of thousands of Israelites from time to time makes little difference to this large deficit.
The biblical historical account of the Exodus also records that the first generation of fighting men experienced abnormally high death rates as a judgment from God for their fear and rebellion when they were supposed to launch the Eviction/Conquest (Numbers 14:26–35). But this judgment seems to have been enacted as a natural consequence of cardiovascular events associated with high intakes of sodium,[3] and would therefore have tended to cull men who had already passed the age range when married couples bear children. So it, too, fails to explain the large number of missing fighting men implied by an anticipated 2.4% per year population growth rate.
A possible answer to the question of why the population growth rate reduced to zero while the Israelites lived in the desert may be found in a study of harmful substances present in natural manna.
Natural manna, we now know, was an efflorescence of salts from the ground, resulting from the combination of stockyard air anions with desert soil cations.
A mixture of minute crystals of principally sodium acetate trihydrate and sodium propionate, but also including trace amounts of other substances such as sodium butyrate, precipitated from solution, forming a fine, white, flake-like efflorescence layer covering the surface of the many acres of ground surrounding the Israelites' city-camp.[4]
Manna was intended to be a short-term solution to the Israelites' need for calories while they journeyed for a year or two across the desert from Egypt to the Promised Land. In such a short-term situation, potentially harmful trace substances in manna would not have been of great concern. The consumption of adequate daily calories afforded by the manna was essential and greatly outweighed the risks associated with short-term exposure to potentially harmful trace substances contained in natural manna.
But when the Israelites failed to launch the Eviction/Conquest according to plan, condemning them to a forty-year stay in the desert, this all changed. Manna was the major staple of their diets. Manna consisted mainly—greater than 99%—of the two substances sodium acetate trihydrate and sodium propionate. These major ingredients are harmless. In fact, they are used as preservatives and flavor ingredients in the food industry today. But natural manna contains other substances in trace amounts, and some of these are known today to be detrimental to health. For this reason, synthetic manna which is manufactured commercially today excludes trace substances. The resulting synthetic manna, while obviously closely approximating the look and taste of natural manna, has the advantage of being free of potentially harmful trace substances.
Harmful trace substances present in natural manna can be identified by examining the chemical compounds present in stockyard air. The first two columns of Table 1 show relative concentrations of volatile organic compounds (VOCs) measured in stockyard air from sheep plus their waste.[5] The relative concentrations are given as the emission ratio (ER) of the specified VOC relative to ammonia in units of parts per trillion of the VOC to parts per billion of ammonia in the sheep stockyard air. This shows immediately that acetic acid is the most abundant VOC in sheep stockyard air, followed by methanol and then ethanol.
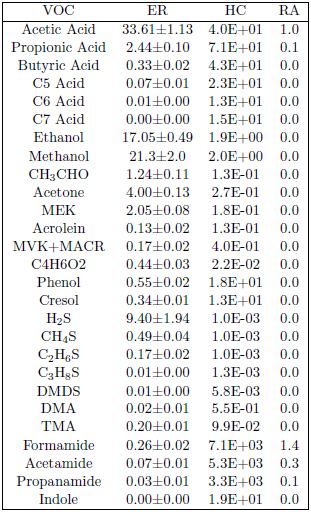 |
To get into natural manna in a significant amount from the air, a compound must have a suitable affinity for water as opposed to air (i.e., it has to have a large enough Henry's constant). Recall that manna fell with the dew overnight. Manna was formed by stockyard gases entering dew, chemically (ionically) reacting in the aqueous solution provided by the dew in contact with the ground, and then appearing as a solid residue left behind on the surface of the ground as the sun evaporated the dew the next morning. Thus, to get into manna, a VOC must have an affinity for water. Henry's constant specifies the affinity of a VOC for the water droplet phase (i.e., the dew) compared to its affinity for staying in the air. The third column (the HC column) of Table 1 shows the approximate Henry's constant for each VOC in the table.[6]
The abundance of sheep stockyard VOCs in manna solution can be calculated by multiplying the ER of a given VOC (which gives its abundance in the air) by Henry's constant (which gives its affinity for water as opposed to air). For ease of comparison, I have shown this as a relative abundance (RA) in the final column of Table 1, where the abundance of each VOC in manna solution has been divided by the abundance of acetic acid in manna solution. This shows immediately that most sheep stockyard VOCs will have no significant abundance in manna solution. In contrast to this general trend, the "amide" compounds stand out, and formamide stands out most conspicuously. It gives a relative abundance in manna solution 40% greater even than that of acetic acid, the main ingredient of manna.
Now I need to pause and clarify some chemistry in an effort to avoid confusing everybody. I am not saying that there will be 40% more formamide molecules than acetic acid molecules in finished manna. Acetic acid molecules behave differently in water than do formamide molecules. Acetic acid molecules ionize (break apart) in water, yielding acetate anions and hydrogen cations. This causes dew to act as a trap for acetic acid molecules, concentrating acetate ions. In contrast, formamide molecules do not ionize in water and thus do not get trapped or concentrated. So manna will be made up mainly of acetate (as sodium acetate trihydrate), not formamide.
But, getting back to the main point, formamide will be present as a significantly abundant trace substance in finished manna. It will be present 1) because its large Henry's constant means it will partition from stockyard air into dew, making it significantly abundant in manna solution, and 2) because formamide, which is an oily liquid at room temperature, has a low vapor pressure, over two hundred times less than the vapor pressure of water at room temperature, meaning that formamide will be left behind as a trace substance on natural manna crystals once the water has evaporated from them. Thus, eating natural manna as a major staple year after year, as the Israelites wound up doing during the Exodus, will necessarily entail chronic ingestion of significant amounts of formamide.
The amount of formamide ingested may be estimated as follows. The concentration of acetic acid in Israelite Negev desert stockyard dew at the surface of the ground has previously been calculated to be 1.2×10-4 mole/L.[7] So the concentration of formamide in the dew will be (1.4 × 1.2×10-4 =) 1.7×10-4 mole/L. Meanwhile, the concentration of sodium acetate trihydrate (SAT) in manna solution has been shown to be roughly 1.5×10-2 mole/L (approximating the composition of manna to be 100% sodium acetate trihydrate).[8] So the mass of formamide eaten per kilogram of natural manna is (1.7×10-4 moles formamide/L × 45 g formamide/mole formamide / 1.5×10-2 mole SAT/L × 136 g SAT/mole SAT =) 3.8×10-3 kilogram formamide.
The consumption of manna was roughly a gallon per person per day. This weighed roughly 1.6 kilograms. So each person was daily consuming up to 6 grams of formamide. This amounts to about a teaspoon of formamide per person per day.
Formamide is not a very nice substance in regard to human exposure. It is definitely not safe for ingestion. It is suspected of causing cancer, but more to the point in the present study, its toxicological profile includes:[9]
Reproductive Effects May cause harm to the unborn child. Possible risk of impaired fertility.
Developmental Effects May cause harm to the unborn child. Developmental effects have occurred in experimental animals.
Teratogenicity Teratogenic effects have occurred in experimental animals.
The biblical historical account of the Exodus reveals that the rate of population growth dropped from around 2.4% per year prior to the Exodus to near zero during the forty years the Israelites spent in the desert following the Exodus. The Israelites ate manna as a dietary staple while they lived in the desert. The manna was provided as a natural efflorescence from desert soil of sodium salts of organic acids found in stockyard air. Modern measurements of other volatile organic compounds found in stockyard air single out formamide as likely the most abundant trace substance present in natural manna. Toxicological studies of formamide reveal that ingestion is harmful to the body and that it may be especially harmful to the body of an unborn child. Of specific interest to the present study, in addition to these harmful effects, formamide is found to be associated with "risk of impaired fertility." This strikes directly at the ability to conceive children. It thus appears that the zero population growth experienced by the Israelites while they lived in the desert may have resulted from the presence of formamide as a harmful trace substance in the natural manna which they ate, dramatically reducing their rate of conception.
In closing, I conjecture that God's purpose in this may have been to prevent the Israelite population from exceeding the carrying capacity of the desert ecosystem in which they dwelt, which otherwise would have resulted in famine. ◇
The Biblical Chronologist is written and edited by Gerald E. Aardsma, a Ph.D. scientist (nuclear physics) with special background in radioisotopic dating methods such as radiocarbon. The Biblical Chronologist has a fourfold purpose: to encourage, enrich, and strengthen the faith of conservative Christians through instruction in biblical chronology and its many implications, to foster informed, up-to-date, scholarly research in this vital field, to communicate current developments and discoveries stemming from biblical chronology in an easily understood manner, and to advance the growth of knowledge via a proper integration of ancient biblical and modern scientific data and principles. The Biblical Chronologist (ISSN 1081-762X) is published by: Aardsma Research & Publishing Copyright © 2023 by Aardsma Research & Publishing. Scripture quotations taken from the (NASB®) New American Standard Bible®, Copyright© 1960, 1971, 1977, 1995 by The Lockman Foundation. Used by permission. All rights reserved. www.Lockman.org |
^ Gerald E. Aardsma, The Exodus Happened 2450 B.C. (Loda, IL: Aardsma Research and Publishing, 2008), 47–48. www.BiblicalChronologist.org.
^ http://en.wikipedia.org/wiki/World_population.
^ Gerald E. Aardsma, Bread from Heaven: The Manna Mystery Solved (Loda, IL: Aardsma Research and Publishing, 2023), 77–80. www.BiblicalChronologist.org.
^ Gerald E. Aardsma, Bread from Heaven: The Manna Mystery Solved (Loda, IL: Aardsma Research and Publishing, 2023), 82. www.BiblicalChronologist.org.
^ Bin Yuan et al., "Supplement of Emissions of volatile organic compounds (VOCs) from concentrated animal feeding operations (CAFOs): chemical compositions and separation of sources" Supplement of Atmospheric Chemistry and Physics, 17 (2017): 4945–4956, Table S7 (sheep).
^ R. Sander: Compilation of Henry's law constants (version 5.0.0) for water as solvent, Atmospheric Chemistry and Physics, 23, 10901-12440 (2023), www.henrys-law.org/henry/.
^ Gerald E. Aardsma, Bread from Heaven: The Manna Mystery Solved (Loda, IL: Aardsma Research and Publishing, 2023), Appendix B, page 96, equation B.18. www.BiblicalChronologist.org.
^ Gerald E. Aardsma, Bread from Heaven: The Manna Mystery Solved (Loda, IL: Aardsma Research and Publishing, 2023), Appendix B, page 101, equation B.39. www.BiblicalChronologist.org.
^ www.fishersci.com/msdsproxy%3FproductName%3DB P227100%26productDescription%3DFORMAMIDE%2BM OL%2BBIO%2BGRADE%2B100ML%26catNo%3DBP227-100%26vendorId%3DVN00033897%26storeId%3D10652 (accessed November 25, 2023).