
 | |
| Volume 15, Number 1 | June 10, 2025 |
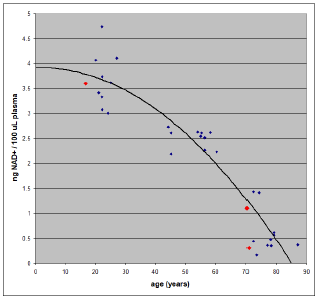
In-house measurements of NAD+ levels in blood plasma (the yellowish liquid which separates out above the aggregated red blood cells when blood is centrifuged) have now revealed that daily intakes of 500 mg nicotinamide riboside (NR) for roughly half a year have failed to restore youthful NAD+ levels in two 70-year-olds (Figure 1). The present article explains the significance of this research finding, provides further theoretical development, and charts a path forward for my present research quest to find a practical means of mitigating residual aging-induced diseases once aging itself has been cured.
 |
Part 1 of this series ended as follows:[1]
I expect to be working out a lab procedure for monitoring NAD levels so daily intake of NR can be adjusted: 1) to optimize NAD concentration in blood plasma, and 2) to minimize NR supplementation expense. This is expected to refine this simple 500-mg-per-day initial recommendation. I will, of course, share what I eventually learn.In the six months which have passed since then, I have been engaged full time in the laboratory developing the apparatus and method necessary to monitor NAD+ levels in human blood plasma. The experimental problem boils down to having to measure a few nanograms of NAD+, a somewhat unstable molecule, in a fraction of a milliliter of human blood plasma, an environment sporting a huge number of other chemical compounds. How does one measure such a tiny quantity of NAD+ in such a complex matrix? Fortunately, others have gone before and shown the way. I followed the lead of J. Clement et al.[2]
These same researchers, in the same article, published a graph of their experimentally determined NAD+ concentrations in human blood plasma versus age. Their data, which were shown in Figure 2 in the first part of this series, are shown again in Figure 1 above because of their importance, being the North Star of the present research endeavor to mitigate aging-induced diseases in the post-aging human.
Two congenital deficiency diseases are the root cause of modern human aging: vitamin MePA deficiency disease and vitamin MePiA deficiency disease.
These two diseases are congenital today because the two vitamins involved are missing from earth's environment at present. They were present before Noah's Flood, which is why Genesis records humans living for more than 900 years in the pre-Flood world. Back then, humans did not die of these vitamin deficiency diseases as we do today. The Flood destroyed the source of these two vitamins, causing human lifespans to dwindle to 70 or 80 years, as they are today and have been for thousands of years.
The restoration of (synthetically produced) vitamins MePA and MePiA to the modern human diet cures both vitamin MePA deficiency disease and vitamin MePiA deficiency disease.[3]
While these cures improve health and increase life expectancy, they do not completely restore youthful health and vigor. Aging-induced diseases remain. In particular, MePiA deficiency disease, active before supplementation with MePiA began, induces a cascade of other diseases.
MePiA is an antioxidant believed to be specific to the mitochondria. Mitochondria are the power-houses of our cells. Through a process known as oxidative phosphorylation, they supply most of the energy that cells need to go about their business.
In the process of generating energy for cells, oxidative phosphorylation produces side products known as reactive oxygen species (ROS). As their name implies, ROS are very chemically reactive. They can engage in unwanted, deleterious chemical reactions with biomolecules. If we think of cells as machines, and biomolecules as the parts making up the machine, then ROS have the unwanted ability to break the parts of the machine, ultimately destroying the machine.
To prevent this, MePiA has the job of extinguishing ROS at the point of their production.
In the absence of MePiA, this vital job is not done. In consequence, out-of-control ("wildfire") damage accumulates over time in our mitochondria. Since the mitochondria are responsible for producing most of the energy our cells need, the result is declining cellular energy as we age. Imagine a battery-powered toy, such as a train. The batteries provide the energy needed for the train to move, flash lights, and make sounds. As the batteries wear down, the train moves more slowly, its lights dim, and the pitch and volume of its sounds are reduced. In the absence of MePiA, the same problem of deficient energy supply is happening to our cells. The result is increasingly dysfunctional cells, and this results in increasingly dysfunctional organs, such as the heart and skeletal muscles, for example. In short, it results in increasing physical deterioration as we age.
The fundamental damage to mitochondria appears to be to its DNA (mtDNA), which contains the blueprint for building the oxidative phosphorylation apparatus within the mitochondria. This kind of damage will result in mutated mtDNA. This kind of mtDNA mutation is called microheteroplasmy. Thus, microheteroplasmy is believed to be the underlying disease responsible for cellular energy starvation.
Microheteroplasmy is thus the first example of an aging-induced disease which persists after aging itself has been cured.
I introduced nicotinamide riboside (NR) as nicotinamide riboside chloride (NR-Cl) into my wife's and my post-aging diets in an effort to combat energy starvation due to microheteroplasmy. The rationale behind this was as follows.
An obvious way to mitigate wildfire ROS production and damage to mitochondria is to slow down oxidative phosphorylation. This appears to be the reason behind the decline in NAD+ with age seen in Figure 1. NAD+ fuels oxidative phosphorylation energy production. Reducing availability of NAD+ in the mitochondria will slow oxidative phosphorylation and thus slow ROS production. This is the NAD+ Destruction Hypothesis previously introduced.
NAD+ Destruction Hypothesis: In the absence of dietary MePiA, ROS-damaged mitochondria deliberately destroy NAD+ to slow further ROS production via oxidative phosphorylation to limit the rate of further ROS damage.
NR is a B3 vitamer (a form of vitamin B3), and vitamin B3 is a precursor to NAD+. Supplementing the diet with NR boosts NAD+, which boosts oxidative phosphorylation, alleviating cellular energy starvation.
This strategy for mitigation of cellular energy starvation makes sense in the post-aging diet because MePiA is no longer absent from that diet, removing the threat of wildfire ROS damage.
To determine whether a strategy to boost NAD+ is succeeding, it is necessary to be able to measure NAD+ concentration in blood plasma. A refrigerated centrifuge was used to separate blood plasma from whole blood. NAD+ is present in plasma only in tiny amounts—less than about 5 nanograms (ng) per 100 microliters (μl) of blood plasma. Measurements at this low level in a sample possessing a complex matrix can be accomplished using an LC-MS-MS apparatus (Figure 2).[4]
 |
Supplementation with 500 mg of NR (as NR-Cl) per day began November 11, 2024 and carried on through the time of measurement, May 16–21, 2025.
Samples were prepared using 200 μl of blood plasma, separated from 500 μl of freshly collected whole blood obtained from a finger prick. Blood samples were taken in the morning, roughly 24 hours after any supplemental NR intake.
NAD+ in the sample was measured relative to a D4 NAD+ isotopic standard via LC-MS-MS using negative parent ion mode.
Three samples were measured (red data points in Figure 1). The first was from myself, yielding 1.1 ng NAD+/100 μl plasma. The second was from a grandson (with parental consent), who has never supplemented NR. The result was 3.6 ng NAD+/100 μl plasma. The third was from my wife. This gave 0.3 ng NAD+/100 μl plasma.
Further measurements will eventually help clarify measurement uncertainties, but for the time being, based on method-development measurements, these seem likely to be at least plus or minus 15% of the measured value, and they probably grow larger than this from 1 toward 0 ng NAD+/100 μl plasma.
The measurement of NAD+ concentration in my blood plasma immediately established that: 1) NAD+ levels in post-aging individuals are deficient, just as is the case with aging individuals, and 2) that youthful NAD+ levels had not been achieved in me by the strategy of supplementation with NR at 500 mg per day for six months.
My grandson's result accomplished two objectives. 1) It corroborated the decline in NAD+ levels with age reported by J. Clement et al. shown in Figure 1. 2) It verified that my LC-MS-MS apparatus and method were working properly.
My wife had been on the same supplementation regimen as I had been. Her result corroborated the conclusion that youthful NAD+ levels had not been achieved by the strategy of supplementation with NR at 500 mg per day for six months.
Ideally, baseline measurements should have been made before beginning dietary supplementation with NR. These would have enabled an assessment of whether any progress toward youthful NAD+ levels had been made. But we had no ability to make such measurements back at that time, and we did not wish to wait because, having both reached 70 years of age, time was clearly of the essence in combating residual, post-aging diseases in both of our cases. As it turned out, we did not miss anything of much importance. Even if there has been some progress toward more youthful NAD+ levels, that progress has nonetheless obviously been far too slow. A better strategy is called for.
Even relatively brief supplementation with NR has been shown to boost NAD+ levels significantly in whole blood (i.e., blood cells plus blood plasma).[5]
Consumption of 100, 300 and 1000 mg NR [per day] dose-dependently and significantly increased whole blood NAD+ (i.e., 22%, 51% and 142%) and other NAD+ metabolites within 2 weeks.Thus, the result of the present study, showing failure to restore more youthful NAD+ levels in blood plasma following six months of NR supplementation at 500 ml NR per day, was not anticipated. How is this result to be understood?
To answer this question, I suggest, at least as a starting point, use of the model shown in Figure 3. It provides an explanation of the present outcome. In this model, NAD+ may be boosted significantly in cells with little effect on levels in blood plasma.
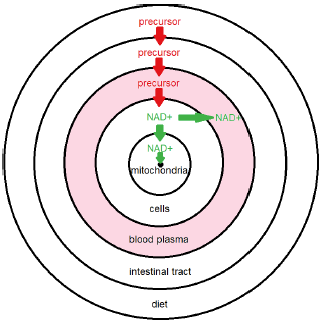 |
The concentric circles represent conceptual domains. The outer circle is the diet domain. It feeds the body with NAD+ precursor—such as natural vitamin B3—which we are augmenting with dietary NR. Precursor enters the bloodstream from the intestinal tract, dissolving in blood plasma. From there, precursor enters the cells domain and is converted to NAD+. Notice that this is the first appearance of NAD+. Precursor does not convert to NAD+ in blood plasma. To get into blood plasma, NAD+ must move from the cells into the plasma, but NAD+ may also move into the mitochondria domain, from which it may be lost via the NAD+ sink domain.
For healthy (i.e., youthful) individuals, the sink is inconsequentially small relative to the flux of NAD+ from the natural diet. NAD+ is then abundant in cells, from which excess NAD+ enters the blood plasma, producing characteristic youthful levels.
Aging disease alters this natural state. It results in enlargement of the NAD+ sink. This results in a substantial flow of NAD+ into the sink. This flow depletes NAD+ in cells, causing them to scavenge NAD+ from blood plasma. Repletion of NAD+ in the blood plasma requires an input of precursor large enough to completely overwhelm the sink.
Now notice that aging-induced microheteroplasmy disease is not a cause of NAD+ depletion. Microheteroplasmy results in dysfunctional mitochondria with consequent depletion of ATP (the energy molecule) and cellular energy starvation, but none of this depletes NAD+.
Depletion of NAD+ is caused by an immune response to the wildfire-ROS-damage of mitochondria in the presence of aging disease (specifically, MePiA deficiency disease). The immune system targets NAD+ for destruction to slow growth of microheteroplasmy and its resultant energy starvation and ultimate death of the organism.
This necessary immune response produces what is, in essence, an autoimmune disease. The immune system is attacking the body's own necessary pool of NAD+. This aging-induced, autoimmune disease, I will call NAD+ autoimmune disease. This is the second aging-induced disease resulting from MePiA deficiency disease.
NAD+ autoimmune disease results in whole-body depletion of NAD+. This is a third aging-induced disease resulting from MePiA deficiency disease. This whole-body depletion I will call NAD+ deficiency disease, in analogy to the vitamin deficiency diseases. This seems appropriate because, just like the traditional vitamins, NAD+ fills multiple physiological roles. When NAD+ is depleted by the immune system to protect the mitochondria, the resulting whole-body deficiency of NAD+ has multiple, wide-ranging consequences for health, just as with the vitamin deficiency diseases.
Thus, I count three aging-induced diseases due to the aging disease MePiA deficiency: 1) microheteroplasmy, 2) NAD+ autoimmune disease, and 3) NAD+ deficiency disease. All three of these diseases persist in the post-aging individual and must be dealt with.
Given these elements of the present theory, it does not seem to make good sense, from a design perspective, for the immune response targeting NAD+ to be a whole-body response. Rather, it seems to make most sense for the destruction of NAD+ to be localized within the mitochondria, to interfere as little as possible with the other physiological roles played by NAD+. This theoretical consideration is the reason I have placed the NAD+ sink domain inside the mitochondria domain in my conceptual model.
Mitochondria are needed to produce energy for cells. Mutated, dysfunctional mitochondria (microheteroplasmy) results in cellular energy starvation.
It might be thought that the immune response to protect mitochondria from ROS damage (NAD+ autoimmune disease) doesn't make much sense because it makes energy starvation worse—it makes worse what it is trying to fix. Exacerbated cellular energy starvation is one of the consequences of NAD+ deficiency disease. NAD+ deficiency slows the rate of oxidative phosphorylation, and this slows ATP production, worsening cellular energy starvation.
But this does make sense. More energy starvation due to NAD+ autoimmune disease makes sense because it results in a longer life expectancy for the organism. It lowers the rate of ROS damage to mitochondria, yielding a longer functional life span for the mitochondria and thus a slower long-term rate of worsening of energy starvation.
The above theory should be regarded as mutable theory, not dogma. Nonetheless, it is helpful in suggesting several lines of attack on the three identified post-aging diseases.
One can imagine various ways to remedy microheteroplasmy. For example, replace all damaged mitochondria with new mitochondria, or edit the mtDNA of all mitochondria to rid them of mutations. So far, medical science does not seem to have achieved the advanced state of technology necessary to make these remedies a practical reality.
Another possibility is to focus on NAD+ autoimmune disease. Shrinking the NAD+ sink to youthful dimensions would allow NAD+ to return to youthful levels. The sink is believed to be made up of CD38 enzymes, which destroy NAD+. Molecules are known which block or inhibit the action of CD38, and some of these may be taken orally. Unfortunately, this is not a care-free route to go because CD38 is necessary for other purposes in the body, and also because CD38 blockers are not native to the body. In addition, it seems unlikely that any CD38 blocker would be able to cross the double membrane into the mitochondria. If the sink for NAD+ is located within the mitochondria, and if CD38 blockers were unable to cross the double membrane into the mitochondria, then CD38 blockers would not be effective against NAD+ autoimmune disease.
So far, NR supplementation seems to be the best strategy. NR is a native body molecule which functions as a vitamer of B3. The body is generally tolerant of larger intakes of vitamins than the natural diet supplies, and NR has been shown to be safe for oral ingestion up to 2000 mg per day. Of the three aging-induced diseases, NAD+ deficiency disease seems likely to be the most damaging to the overall health of a post-aging individual. NR fights NAD+ deficiency disease directly, and in doing so, it also combats energy starvation, mitigating microheteroplasmy disease.
As seen above, NR supplementation, at a level even as low as 100 mg per day, is known to boost NAD+ levels in whole blood. This means that red blood cells, at least, are being made less deficient in NAD+ by even low-level NR supplementation. No doubt, this is true of many other types of cells throughout the body as well. So supplementing NR at even insufficient levels is still helpful to overall health. The trend of slow improvement of my own health over the six months of the present experiment supports this conclusion. But how is one to determine when sufficient supplementation with dietary NR has been achieved?
It seems appropriate to say that until youthful levels of NAD+ in blood plasma have been attained, NAD+ deficiency disease has not been cured. The goal, therefore, is to boost blood plasma back to youthful levels and keep it there. The purpose of the present research is to determine how much NR is required to reach this goal.
The model presented above suggests that blood plasma may well be the last domain to get replenished once supplementation with NR begins. Since blood plasma does no convert NR to NAD+, it is dependent on cells for its supply of NAD+, and cells may hang on to whatever NAD+ they can produce, until their needs have been satisfied, before sending excess into the blood plasma.
Taking the theoretical model a step further emphasizes the importance of supplementing with sufficient NR to restore NAD+ in blood plasma to youthful levels. It seems unlikely that the youthful presence of NAD+ in blood plasma has no purpose other than to serve as a NAD+ reserve. Once again from a design perspective, the presence of NAD+ in blood plasma would allow some special cells to be free of the need to convert precursor to NAD+, drawing their NAD+ exclusively from the blood plasma pool. I have no idea whether such special cells exist in the body, but if they do, it follows that NAD+ deficiency disease would be especially detrimental to their function. Thus, no matter how replenished with NAD+ some types of cells may appear to be, NR supplementation must be regarded as insufficient until such time as youthful levels of NAD+ have been obtained in blood plasma.
NR-Cl supplementation at 500 mg per day for six months has failed to restore youthful blood plasma levels of NAD+ in both a male and a female 70-year-old. Present theory suggests that the best path forward at this juncture is merely to increase NR intake. Accordingly, the present research strategy is to double the daily intake of NR to 1000 mg per day for a month or more and see what effect this has on blood plasma concentrations of NAD+. ◇
The Biblical Chronologist is written and edited by Gerald E. Aardsma, a Ph.D. scientist (nuclear physics) with special background in radioisotopic dating methods such as radiocarbon. The Biblical Chronologist has a fourfold purpose:
The Biblical Chronologist (ISSN 1081-762X) is published by: Aardsma Research & Publishing Copyright © 2025 by Aardsma Research & Publishing. |
^ Gerald E. Aardsma, "Dietary Supplementation with NR for the Post-aging Diet," The Biblical Chronologist 14.17 (December 12, 2024): 1–7. www.BiblicalChronologist.org.
^ James Clement et al., "The Plasma NAD+ Metabolome Is Dysregulated in 'Normal' Aging. Rejuvenation Research 22, 2 (April 23, 2019): 121–130. https://pmc.ncbi.nlm.nih.gov/articles/PMC6482912/
^ Gerald E. Aardsma, Aging: Cause and Cure, 3rd ed. (Loda, IL: Aardsma Research and Publishing, 2023). www.BiblicalChronologist.org.
^ A PBr column was used for HPLC, following the lead of Makoto Ozaki, Motoshi Shimotsuma, and Tsunehisa Hirose, "Separation of Highly Hydrophilic Nicotinamide Metabolites Using a COSMOSIL PBr Column" MethodsX 10 (February 2, 2023): 1–6. www.sciencedirect .com/science/article/pii/S221501612300064X
^ Dietrich Conze, Charles Brenner, and Claire L. Kruger, "Safety and Metabolism of Long-term Administration of NIAGEN (Nicotinamide Riboside Chloride) in a Randomized, Double-Blind, Placebo-controlled Clinical Trial of Healthy Overweight Adults" Scientific Reports 9.9772 (2019): 1–13. https://www.nature.com/articles/s41598-019-46120-z